Bisantrene dihydrochloride
Synonym(s):Bis((4,5-dihydro-1H-imidazol-2-yl)hydrazone)-9,10-anthracenedicarboxaldehyde dihydrochloride;NSC 337766
- CAS NO.:71439-68-4
- Empirical Formula: C22H22N8.2(HCl)
- Molecular Weight: 471.39
- MDL number: MFCD01743164
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-07-02 08:55:01
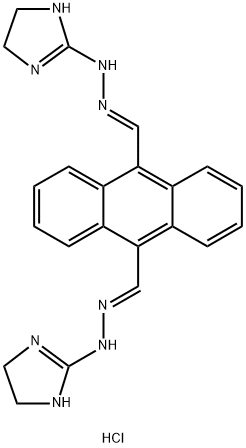
What is Bisantrene dihydrochloride?
Description
Bisantrene hydrochloride is a cytostatic useful in the treatment of adult acute, nonlymphocytic leukemia. It is reportedly active against lymphoma, myeloma, bladder, lung and advanced breast cancer, but not small-cell lung cancer, renal cancer, melanoma or refractory adult acute leukemia.
Originator
Lederle (USA)
The Uses of Bisantrene dihydrochloride
Antineoplastic.
Manufacturing Process
A 33.0 g (0.135 mole) of 2-methylthio-2-imidazoline hydroiodide is dissolved
in 300 ml of water and treated with 8 ml (0.16 mole) of hydrazine hydrate.
The mixture is stirred at room temperature for 20 hours and then taken to
dryness under reduced pressure. The residue is dissolved in 250 ml of water
and again taken to dryness under reduced pressure. The residue is
redissolved in 250 ml of water and added to a mixture of 250 ml of water, 25
ml of concentrated hydrochloric acid and 25 g of silver oxide. The resulting
mixture is stirred on a steam bath for 4 hours and then filtered. The filtrate is
reduced to dryness under reduced pressure. The residue is dissolved in 300
ml of ethanol and 20 ml of water at the boil, clarified and cooled at -10°C.
The precipitate is collected, washed with ethanol and ether and dried at 60°C
and then 110°C under reduced pressure. Yield of the 2-hydrazino-2-
imidazoline hydrochloride 11.6 g, melting point 177-180°C.
The 2-hydrazino-2-imidazoline monohydrochloride is converted to the
dihydrochloride by treatment with ethanol and concentrated hydrochloric acid.
A suspension of 3.46 g of the 2-hydrazino-2-imidazoline dihydrochloride and
2.34 g of 9,10-anthracenedicarboxaldehyde in 100 ml of ethanol is stirred and
heated under reflux for two hours. The mixture is cooled and the solid is
collected and washed with ethanol giving the desired product as a crystalline
orange solid, m.p. 288-289°C (dec.).
brand name
Zantrene
Therapeutic Function
Antineoplastic
Safety Profile
Mutation data reported. Whenheated to decomposition it emits toxic fumes of NOx andHCl.
Properties of Bisantrene dihydrochloride
| Melting point: | 288-289° (dec) |
| form | Solid |
| color | Orange crystals |
Safety information for Bisantrene dihydrochloride
Computed Descriptors for Bisantrene dihydrochloride
New Products
Mirtazapine Impurity C/Mirtazapine Lactam Impurity N,O-Dimethylhydroxylamine hydrochloride Tetrabutylammonium perchlorate N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid (R)-1-Benzyl-3-pyrrolidinecarbonitrile N-Nitroso hydroxy Cetrizine EP Impurity-A Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity Lantanoprost rc B Clidinium Bromide Impurity 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 3,4 Diethoxy Benzylcyanide 2-Chloro Benzylcyanide 3-chlorobenzyl cyanide 3,4 Dimethoxy Benzylcyanide valeronitrile 4-Bromo BenzylcyanideRelated products of tetrahydrofuran

You may like
-
 71439-68-4 9,10-bis((Z)-(2-(4,5-dihydro-1H-imidazol-2-yl)hydrazono)methyl)anthracene dihydrochloride (Bisantrene dihydrichloride) 98%View Details
71439-68-4 9,10-bis((Z)-(2-(4,5-dihydro-1H-imidazol-2-yl)hydrazono)methyl)anthracene dihydrochloride (Bisantrene dihydrichloride) 98%View Details
71439-68-4 -
 Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
2517968-40-8 -
 Acebutolol EP Impurity K NLT 95%View Details
Acebutolol EP Impurity K NLT 95%View Details
74143-33-2 -
 Clidinium Bromide Impurity NLT 95%View Details
Clidinium Bromide Impurity NLT 95%View Details
.6581-06-2 -
 192110-67-2 NLT 95%View Details
192110-67-2 NLT 95%View Details
192110-67-2 -
 Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
59872-62-1 -
 .2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 -
 145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1
