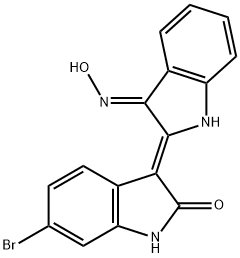
BIO
Synonym(s):BIO, (2ʹ Z,3ʹ E)-6-Bromoindirubin-3ʹ-oxime;BIO, (2ʹZ,3ʹE)-6-Bromoindirubin-3ʹ-oxime;GSK-3 Inhibitor IX - CAS 667463-62-9 - Calbiochem;InSolution GSK-3 Inhibitor IX - CAS 667463-62-9 - Calbiochem
- CAS NO.:667463-62-9
- Empirical Formula: C16H10BrN3O2
- Molecular Weight: 356.17
- MDL number: MFCD08705318
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-31 10:09:29
What is BIO?
Description
BIO is a cell-
The Uses of BIO
6-bromoindirubin-3′-oxime (BIO) has been used:
- in MTT 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) cell proliferation assay
- as a medium supplement in embryonic stem cells (ESCs)
- for the inhibition of glycogen synthase kinase 3 β (GSK-3β) in human dermal papilla cells (hDPCs)
What are the applications of Application
GSK-3 Inhibitor IX is a cell-permeable, highly potent, selective, reversible, and ATP-competitive inhibitor of GSK-3α and GSK-3β.
Definition
ChEBI: 6-bromoindirubin-3'-oxime is a member of the class of biindoles that is indirubin substituted at position 6 by a bromo group and in which the keto group at position 3' has undergone condensation with hydroxylamine to form the corresponding oxime. It has a role as an EC 2.7.11.1 (non-specific serine/threonine protein kinase) inhibitor and an EC 2.7.11.26 (tau-protein kinase) inhibitor. It is a ketoxime, an organobromine compound, a member of oxindoles and a biindole.
General Description
A cell-permeable, highly potent, selective, reversible, and ATP-competitive inhibitor of GSK-3α/β (IC50 = 5 nM). Specificity was confirmed using various Cdk′s (IC50 = 83 nM for Cdk5/p25, 300 nM for Cdk2/cyclin A, 320 nM for Cdk1/cyclin B, and 10 μM for Cdk4/cyclin D1), MAP kinases, PKA, PKC isoforms, PKG, CK, and IRTK (IC50 ≥ 10 μM). Inhibition of GSK by BIO has been shown to result in the activation of the Wnt signaling pathway and sustained pluripotency of human and murine embryonic stem cells (ESCs).
Biological Activity
Potent and selective, ATP-competitive glycogen synthase kinase-3 (GSK-3) inhibitor (IC 50 values are 5, 83, 300, 320 and > 10 000 nM at GSK-3, CDK5/p25, CDK2/cyclin A, CDK1/cyclin B and other common kinases respectively). Maintains self-renewal and pluripotency in embryonic stem cells via activation of the Wnt signaling pathway in vitro .
Biochem/physiol Actions
Cell permeable: yes
Enzyme inhibitor
These protein kinase inhibitors (FWBIO = 356.19 g/mol; CAS 667463-62-9; Solubility: 70 mg/mL DMSO, <1mg/mL H2O), also known as BIO, 6BIO, and systematically as 6-bromo-3-[(3E)-1,3-dihydro-3-(hydroxyimino)-2Hindol- 2-ylidene]-1,3-dihydro-(3Z)-2H-indol-2-one, targets glycogen synthase kinase-3 (or GSK-3), with an IC50 value of 5 nM for α and β forms. BIO also shows greater than 16x selectivity over CDK5 and is also a weaker pan-JAK inhibitor, with IC50 values of 30 nM, 1.5 μM, 8.0 μM, and 0.5 μM for TYK2, JAK1, JAK2 and JAK3. (See the structural related inhibitor 1-Azakenpaullone) BIO, but not 1-methyl-BIO, closely mimicked Wnt signaling in Xenopus embryos. BIO-induced apoptosis of human melanoma cells is associated with reduced phosphorylation of JAKs and STAT3 in both dose- and time-dependent manners. Consistent with inhibition of STAT3 signaling, expression of the anti-apoptotic protein Mcl- 1 was down-regulated. Maintaining Embryonic Stem Cells Pluripotency: Human and mouse embryonic stem cells (ESCs) self-renew indefinitely while maintaining the ability to generate all three germ-layer derivatives. Importantly, Wnt pathway activation by BIO is sufficient to maintain the undifferentiated phenotype in both types of ESCs and sustains expression of the pluripotent state-specific transcription factors Oct-3/4, Rex-1 and Nanog. Such results suggest that BIO and related GSK-3-specific inhibitors may off practical advantages in regenerative medicine. Bio can also participate in controlling the proliferative capability of the highly differentiated cardiomyocytes. Activation of Wnt/β-catenin and inhibition of Notch signaling pathways, as mediated by simultaneous co-application of BIO and the γ-secretase inhibitor N-[(3,5-difluorophenyl)acetyl]-L-alanyl-2- phenylglycine-1,1-dimethylethyl ester (or DAPT), efficiently induces intestinal differentiation of ESCs cultured on feeder cells. These findings that Wnt and Notch signaling function to pattern the anterior-posterior axis of the DE and control intestinal differentiation. 6-Bromoindirubin-3'- acetoxime: This cell-permeable BIO analogue (FWBIO-acetoxime = 398.21 g/mol; CAS 667463-85-6) is active against herpes simplex virus-1 (HSV-1) infection in human oral epithelial cells, the latter representing a natural target cell type. BIO-acetoxime reduces viral yields and the expression of different classes of viral proteins. BIO-acetoxime may suppress viral gene expression and protect oral epithelial cells from HSV-1 infection. Tyrian Purple: BIO’s indirubin nucleus is related to the famous Tyrian purple dye that the Phoenicians isolated from the gastropod mollusk Hexaplex trunculus and gained favor in antiquity, because it did not fade, actually becoming more brilliant upon weathering and exposure to sunlight. The inhibitory properties of BIO suggest that 6-bromoindirubin provides a new scaffold for the development of selective and potent pharmacological inhibitors of GSK- 3.
in vivo
study showed bio activated maternal wnt signaling pathway in xenopus embryos. it caused a hyper dorso-anteriorization at the expense of trunk and tail in a dose-response manner. it also activated the dorsal genes (siamois and chordin) ectopically. [1]
Storage
+4°C
References
1) Meijer et al. (2003), GSK-3-selective inhibitors derived from Tyrian purple indirubins; Chem. Biol., 10 1255 2) Tseng et al. (2006), The GSK-3 inhibitor BIO promotes proliferation in mammalian cardiomyocytes; Chem. Biol., 13 957 3) Chebel et al. (2009), Indirubin derivatives inhibit malignant lymphoid cell proliferation; Leuk. Lymphoma, 50 2049
Properties of BIO
| Melting point: | 300°C(lit.) |
| Boiling point: | 554.3±50.0 °C(Predicted) |
| Density | 1.80±0.1 g/cm3(Predicted) |
| RTECS | NM3241900 |
| storage temp. | 2-8°C |
| solubility | DMSO: >5 mg/mL |
| form | solid |
| pka | 9.56±0.20(Predicted) |
| color | dark red |
| Sensitive | Air & Light Sensitive |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 2 months. |
Safety information for BIO
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for BIO
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 1-Boc-4-cyanopiperidine R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) Methyl 6-methylnicotinate TETRAHYDRO-2H-PYRAN-3-OL tert-Butyl carbazate 2-Methoxyphenothiazine N-methyl -4-piperidone 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 1-methylindoline-2,3-dione methyl 4-ethyl-3-ethynylbenzoate Methyl phenyl sulfoneRelated products of tetrahydrofuran

![2,6-PYRIDINEDIAMINE, N6-[2-[[4-(2,4-DICHLOROPHENYL)-5-(1H-IMIDAZOL-1-YL)-2-PYRIMIDINYL]AMINO]ETHYL]-3-NITRO-](https://img.chemicalbook.in/CAS/GIF/252935-94-7.gif)
You may like
-
 (2'Z,3'E)-6-Bromoindirubin-3'-oxime CAS 667463-62-9View Details
(2'Z,3'E)-6-Bromoindirubin-3'-oxime CAS 667463-62-9View Details
667463-62-9 -
 BIO CAS 667463-62-9View Details
BIO CAS 667463-62-9View Details
667463-62-9 -
 GSK-3 Inhibitor IX CAS 667463-62-9View Details
GSK-3 Inhibitor IX CAS 667463-62-9View Details
667463-62-9 -
 GSK-3 Inhibitor IX CAS 667463-62-9View Details
GSK-3 Inhibitor IX CAS 667463-62-9View Details
667463-62-9 -
 65497-29-2 99%View Details
65497-29-2 99%View Details
65497-29-2 -
 18979-61-8 99%View Details
18979-61-8 99%View Details
18979-61-8 -
 3-acetyl pyridine 99%View Details
3-acetyl pyridine 99%View Details
350-03-8 -
 66422-95-5 99%View Details
66422-95-5 99%View Details
66422-95-5
