BIA 2-093
- CAS NO.:236395-14-5
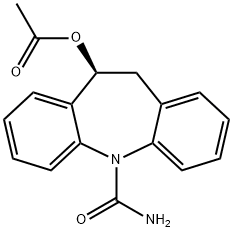
- Empirical Formula: C17H16N2O3
- Molecular Weight: 296.32
- MDL number: MFCD06798333
- EINECS: 636-617-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-02-02 18:10:39
What is BIA 2-093?
Absorption
Eslicarbazepine active metabolite has a high bioavailability and reaches peak serum concentration 1-4 hours after a given dose. Eslicarbazepine acetate absorption is not affected by food.
Toxicity
There are no adequate and well-controlled studies of the use of eslicarbazepine acetate in pregnant women. In studies conducted in pregnant mice, rats, and rabbits, eslicarbazepine acetate did show developmental toxicities, including teratogenicity, embryolethality, and fetal growth retardation, at clinically relevant doses. Drug-induced liver injury ranging from mild to moderate elevations in transaminases (>3 times the upper limit of normal) to rare cases with concomitant elevations of total bilirubin (>2 times the upper limit of normal) have been reported with the use of eslicarbazepine. Overdose with eslicarbazepine acetate appears similar to its known adverse reactions and includes symptoms of hyponatremia, dizziness, nausea, vomiting, somnolence, euphoria, oral paraesthesias, ataxia, and diplopia. There is no specific antidote for eslicarbazepine acetate overdose and it should be treated primarily with supportive measures. If required, the drug may be removed by gastric lavage, partially by hemodialysis or inactivated with activated charcoal.
Description
Although epilepsy is a neurological disorder with varying etiology and severity, the common feature is unprovoked, recurring seizures. Even when traditional AEDs, such as phenobarbital, phenytoin, carbamazepine, and valproate, control seizure activity, their effectiveness is hampered by considerable side effects and the tendency for drug-drug interactions. Designed for improved efficacy and safety, eslicarbazepine acetate is a third-generation AED that has been approved as adjunctive therapy for partial-onset seizures with or without secondary generalization. As a member of the carbamazepine family (first generation), it is the prodrug of eslicarbazepine and the active metabolite of oxcarbazepine (second generation). .
Description
Eslicarbazepine acetate is a sodium channel blocker (IC50 = 138 nM in a radioligand binding assay). It inhibits sodium uptake in a dose-dependent manner in rat cortical synaptosomes at concentrations ranging from 30-300 μM. In vivo, oral and i.p. administration of eslicarbazepine acetate is protective against seizures induced by maximal electroshock (MES) in mice with ED50 values of 4.7 and 6.3 mg/kg, respectively, which are well below the median toxic dose (TD50) values of 358.7 and 78.6 mg/kg for oral and i.p. administration respectively. High-dose administration (30 mg/kg) of eslicarbazepine acetate prevents picrotoxin-induced seizures in rats. Low-dose administration (10 mg/kg) does not suppress picrotoxin-induced seizures, however, it reduces seizure number and duration. Formulations containing eslicarbazepine acetate have been used for the treatment of partial-onset seizures.
Chemical properties
White to Off-White Solid
Originator
BIAL Group (Spain)
The Uses of BIA 2-093
Eslicarbazepine acetate, (BIA 2-093), is a promising antiepileptic drug structurally related to Carbamazepine and Oxcarbazepine.
The Uses of BIA 2-093
Eslicarbazepine acetate, (BIA 2-093), is a promising antiepileptic drug structurally related to Carbamazepine and Oxcarbazepine. Neuroprotective & Neuroresearch product.
Background
Eslicarbazepine acetate (ESL) is an anticonvulsant medication approved for use in Europe, the United States and Canada as an adjunctive therapy for partial-onset seizures that are not adequately controlled with conventional therapy. Eslicarbazepine acetate is a prodrug that is rapidly converted to eslicarbazepine, the primary active metabolite in the body. Eslicarbazepine's mechanism of action is not well understood, but it is known that it does exert anticonvulsant activity by inhibiting repeated neuronal firing and stabilizing the inactivated state of voltage-gated sodium channels, thus preventing their return to the activated state during which seizure activity can occur.
Eslicarbazepine acetate is marketed as Aptiom in North America and Zebinix or Exalief in Europe. It is available in 200, 400, 600, or 800mg tablets that are taken once daily, with or without food. Eslicarbazepine acetate is associated with numerous side effects including dizziness, drowsiness, nausea, vomiting, diarrhea, headache, aphasia, lack of concentration, psychomotor retardation, speech disturbances, ataxia, depression and hyponatremia. It is recommended that patients taking eslicarbazepine acetate be monitored for suicidality.
Indications
Eslicarbazepine acetate is indicated for the treatment of partial-onset seizures in patients 4 years of age and older.
What are the applications of Application
BIA 2-093 is a voltage-gated sodium channel blocker
Definition
ChEBI: The acetate ester, with S configuration, of licarbazepine. An anticonvulsant, it is approved for use in Europe and the United States as an adjunctive therapy for epilepsy.
brand name
Zebinix; Exalief, Stedesa
Pharmacokinetics
Eslicarbazepine acetate is associated with a dose- and concentration-dependant increase in heart rate and prolongation of PR interval.
Side Effects
The most common adverse events occurring with an incidence of >2% were dizziness, somnolence, nausea, diplopia, headache, vomiting, abnormal coordination, blurred vision, vertigo, and fatigue. While most of these side effects appeared to be mild to moderate, they did display a dose dependency. Although eslicarbazepine acetate is only contraindicated in patients with a known hypersensitivity to the carbamazepine family of AEDs, caution should be exercised in patients with cardiac conduction abnormalities (potential for prolongations in PR intervals), renal impairment, and in patients of Han Chinese and Thai origin since the presence of HLA-B*1502 allele in these individuals has been shown to be strongly associated with the risk of developing Stevens-Johnson syndrome. Regarding drug interactions, eslicarbazepine acetate may decrease the effectiveness of hormonal contraceptives, so alternative or additional methods of contraception should be considered.
Metabolism
Eslicarbazepine acetate is rapidly and extensively metabolized to its major active metabolite, eslicarbazepine, via hydrolytic first-pass metabolism. Eslicarbazepine corresponds to about 92% of systemic exposure. Minor active metabolites (R)-licarbazepine and oxcarbazepine consist of <5% of systemic exposure. Active metabolites are then metabolized to inactive glucuronides that correspond to about 3% of systemic exposure.
Eslicarbazepine had a moderate inhibitory effect on CYP2C19 and a mild activation of UGT1A1-mediated glucuronidation when studied in human hepatic microsomes. It has been shown to induce CYP3A4 enzymes in vivo.
Properties of BIA 2-093
| Melting point: | 183-185°C |
| Boiling point: | 427.4±55.0 °C(Predicted) |
| Density | 1.32 |
| storage temp. | Sealed in dry,2-8°C |
| solubility | H2O: insoluble |
| form | solid |
| pka | 13.97±0.40(Predicted) |
| color | white |
Safety information for BIA 2-093
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for BIA 2-093
| InChIKey | QIALRBLEEWJACW-INIZCTEOSA-N |
BIA 2-093 manufacturer
Besil Chem LLP
Pharma Links
New Products
Mirtazapine Impurity C/Mirtazapine Lactam Impurity N,O-Dimethylhydroxylamine hydrochloride Tetrabutylammonium perchlorate N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid (R)-1-Benzyl-3-pyrrolidinecarbonitrile N-Nitroso hydroxy Cetrizine EP Impurity-A Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity Lantanoprost rc B Clidinium Bromide Impurity 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 3,4 Diethoxy Benzylcyanide 2-Chloro Benzylcyanide 3-chlorobenzyl cyanide 3,4 Dimethoxy Benzylcyanide valeronitrile 4-Bromo BenzylcyanideRelated products of tetrahydrofuran


You may like
-
 Eslicarbazepine Acetate 236395-14-5 98%View Details
Eslicarbazepine Acetate 236395-14-5 98%View Details
236395-14-5 -
 236395-14-5 98%View Details
236395-14-5 98%View Details
236395-14-5 -
 Eslicarbazepine Acetate 236395-14-5 98%View Details
Eslicarbazepine Acetate 236395-14-5 98%View Details
236395-14-5 -
 236395-14-5 Eslicarbazepine Acetate 99%View Details
236395-14-5 Eslicarbazepine Acetate 99%View Details
236395-14-5 -
 236395-14-5 98%View Details
236395-14-5 98%View Details
236395-14-5 -
 BIA 2-093 >98% (HPLC) CAS 236395-14-5View Details
BIA 2-093 >98% (HPLC) CAS 236395-14-5View Details
236395-14-5 -
 Eslicarbazepine Acetate CAS 236395-14-5View Details
Eslicarbazepine Acetate CAS 236395-14-5View Details
236395-14-5 -
 BIA 2-093 CAS 236395-14-5View Details
BIA 2-093 CAS 236395-14-5View Details
236395-14-5
