BETA-LAPACHONE
- CAS NO.:4707-32-8
- Empirical Formula: C15H14O3
- Molecular Weight: 242.27
- MDL number: MFCD01712233
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-28 14:58:11
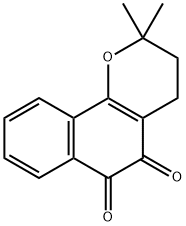
What is BETA-LAPACHONE?
Description
β-Lapachone (4707-32-8) is a naturally occurring quinone found in the bark of the Lapacho tree (Tabebuia avellanedae). A novel DNA topoisomerase I inhibitor which unlike camptothecin does not stabilize the cleavable complex indicating a novel mode of action.1?Induces apoptosis in a number of cancer cell lines.2?In cancer cells over-expressing NAD(P)H:quinone oxidoreductase, reduction of β-lapachone leads to futile cycling between quinone and hydroquinone forms3?resulting in the production of reactive oxygen species4. Suppresses radiation-induced activation of NFκB.5
Chemical properties
Orange Solid
The Uses of BETA-LAPACHONE
β-Lapachone is a naturally occurring quinone obtained from the bark of the lapacho tree (Tabebuia avellanedae) with cancer chemopreventive properties. Induces apoptosis in HL-60 and human prostate cancer cells.
The Uses of BETA-LAPACHONE
β-Lapachone has been used:
- as an anticancer compound in catalase-inhibitable luminol/hydrogen peroxide (HRP)-dependent chemiluminometric?assay in Lewis lung carcinoma (LLC) cells and isolated mitochondria
- as a naphthoquinone to study its effects on the growth and differentiation of mice granulocyte and macrophage progenitor cells
- as a substrate to study the enzyme activity of human recombinant NAD(P)H dehydrogenase 1 (NQO1) protein
What are the applications of Application
β-Lapachone is a selective DNA topoisomerase I inhibitor
Definition
ChEBI: A benzochromenone that is 3,4-dihydro-2H-benzo[h]chromene-5,6-dione substituted by geminal methyl groups at position 2. Isolated from Tabebuia avellanedae, it exhibits antineoplastic and anti-inflammatory activi ies.
Biochem/physiol Actions
β-Lapachone acts as a DNA topoisomerase type I inhibitor. It exhibits anti-fungal, anti-bacterial, trypanocidal, and antiviral properties. β-Lapachone also inhibits nitric oxide?(NO) and inducible NO synthase (iNOS) in alveolar macrophages.
References
1) Li?et al.?(1993),?beta-Lapachone, a Novel DNA Topoisomerase I Inhibitor With a Mode of Action Different From Camptothecin; J. Biol. Chem.,?268?22463 2) Wuerzberger?et al.?(1998),?Induction of Apoptosis in MCF-7:WS8 Breast Cancer Cells by Beta-Lapachone; Cancer Res.,?58?1876 3) Pink?et al.?(2000),?NAD(P)H:Quinone Oxidoreductase Activity Is the Principal Determinant of Beta-Lapachone Cytotoxicity. J. Biol. Chem.,?275?5416 4) Siegel?et al. (2012),?NAD(P)H:quinone Oxidoreductase 1 (NQO1) in the Sensitivity and Resistance to Antitumor Quinones; Biochem. Pharmacol.,?83?1033 5) Dong?et al.?(2010), Beta-lapachone suppresses radiation-induced activation of nuclear factor-kappaB; Exp. Mol. Med.,?42?327
Properties of BETA-LAPACHONE
| Melting point: | >110°C (dec.) |
| Boiling point: | 381.4±42.0 °C(Predicted) |
| Density | 1.25±0.1 g/cm3(Predicted) |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | Soluble in DMSO (up to 35 mg/ml) or in Ethanol (up to 15 mg/ml) |
| form | Orange to brown solid. |
| color | Orange |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 3 months.Shipping Code |
Safety information for BETA-LAPACHONE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for BETA-LAPACHONE
| InChIKey | QZPQTZZNNJUOLS-UHFFFAOYSA-N |
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran
You may like
-
 β-Lapachone CAS 4707-32-8View Details
β-Lapachone CAS 4707-32-8View Details
4707-32-8 -
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 2856-63-5 99%View Details
2856-63-5 99%View Details
2856-63-5 -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5 -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8