BAZEDOXIFENE ACETATE
Synonym(s):1-[4-(2-Azepan-1-yl-ethoxy)benzyl]-2-(4-hydroxyphenyl)-3-methyl-1H-indol-5-ol acetate;TSE-424;WAY-140424;WAY-TSE-424
- CAS NO.:198481-33-3
- Empirical Formula: C32H38N2O5
- Molecular Weight: 530.67
- MDL number: MFCD09260074
- EINECS: 638-804-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:23:56
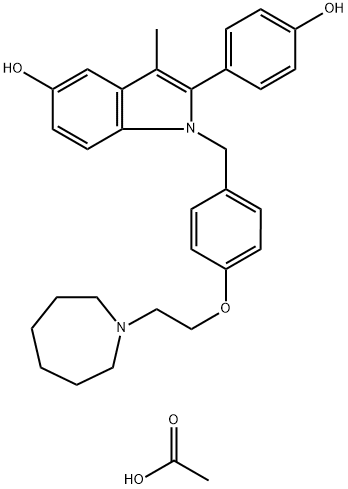
What is BAZEDOXIFENE ACETATE?
Chemical properties
Pale Beige Solid
The Uses of BAZEDOXIFENE ACETATE
Bazedoxifene Acetate is a nonsteroidal selective estrogen receptor modulator (SERM). Bazedoxifene Acetate is used as an antiosteoporotic.
The Uses of BAZEDOXIFENE ACETATE
Bazedoxifene acetate has been used to study its efficacy in inhibiting the interleukin-6 (IL-6)/IL-6R/glycoprotein 130 pathway and its effects on medulloblastoma cells.
Biological Activity
bazedoxifene, a novel selective estrogen receptor modulator (serm), has been developed to have favorable effects on bone and the lipid profile while minimizing stimulation of uterine or breast tissues. two large phase iii
Biochem/physiol Actions
Bazedoxifene is a third generation nonsteroidal selective estrogen receptor modulator (SERM), used clinically to treat postmenopausal osteoporosis. Bazedoxifene binds to estrogen receptor-α with IC50 = 26 nM, similar to that of raloxifene, but lower affinity than 17-β estradiol. Bazedoxifene did not stimulate proliferation of MCF-7 cells, instead inhibited 17β -estradiol-induced proliferation with IC50 = 0.19 nM, exhibiting a desirable profile of agonist/antagonist activity.
Clinical Use
The selective estrogen receptor modulator bazedoxifene acetate was approved in Spain for the treatment of osteoporosis in postmenopausal women. The drug was discovered by Wyeth (now Pfizer) and licensed to Almirall. Clinical trials with bazedoxifene along with conjugated estrogens demonstrated significant improvement in bone mineral density and prevented bone loss in postmenopausal women without osteoporosis. It also reduces fracture risks among women with postmenopausal osteroporosis.
Synthesis
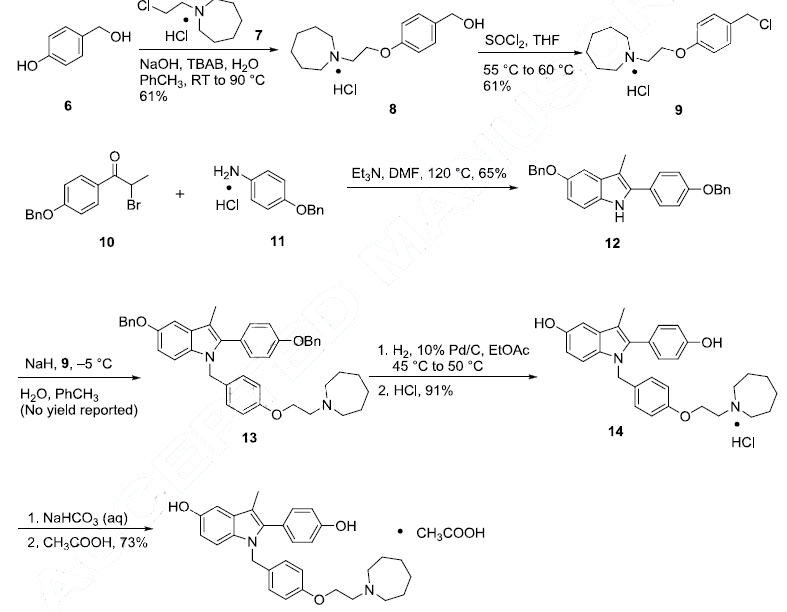
Among many syntheses reported for this drug, the most recent process scale synthesis (multi-kg scale) is highlighted and involves the union of azepane ether 9 and indole 12. 4-Hydroxybenzyl alcohol (6) was converted in two steps to chloride 9 (the Scheme). The reaction of 6 with 2-chloroethyl azepane hydrochloride (7) in a biphasic mixture of sodium hydroxide and toluene in the presence of tetrabutylammonium bromide (TBAB) gave the desired intermediate alcohol 8 in 61% yield. Treatment of 8 with thionyl chloride (SOCl2) gave the requisite chloride 9 in 61% yield. The reaction of 2-bromopropiophenone (10) with an excess of 4-benzyloxy aniline hydrochloride (11) in the presence of triethylamine (TEA) in N,N-dimethylformamide (DMF) at elevated temperatures resulted in indole 12 in 65% yield. Alkylation of 12 with benzylchloride 9 in the presence of sodium hydride (NaH) afforded N-alkylated compound 13. The benzyl ether functionalities from compound 13 were removed via hydrogenolysis and subsequently subjected to acidic conditions, providing diol 14 as the hydrochloride salt in 91% yield. The hydrochloride was then exchanged for the acetate via free base preparation with 5% sodium bicarbonate or triethylamine, followed by treatment with acetic acid giving bazedoxifene acetate (II) in 73¨C85% yield.
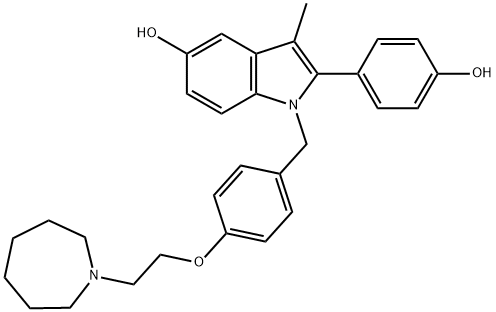
Properties of BAZEDOXIFENE ACETATE
| Melting point: | 174-178° |
| storage temp. | -20°C |
| solubility | DMSO: soluble15mg/mL, clear |
| form | powder |
| color | white to beige |
Safety information for BAZEDOXIFENE ACETATE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for BAZEDOXIFENE ACETATE
| InChIKey | DGEPGJZKMFNSDD-UHFFFAOYSA-N |
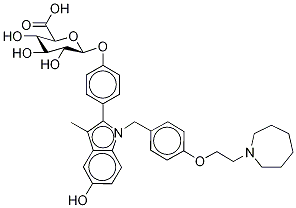
| SMILES | C(=O)(O)C.C(C1C=CC(OCCN2CCCCCC2)=CC=1)N1C2C=CC(O)=CC=2C(C)=C1C1C=CC(O)=CC=1 |
BAZEDOXIFENE ACETATE manufacturer
Archerchem Healthcare Pvt., Ltd. (part of Archerchem Group)
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran
![1-[[4-[2-(Hexahydro-1-oxido-1H-azepin-1-yl)ethoxy]phenyl]Methyl]-2-(4-hydroxyphenyl)-3-Methyl-1H-indol-5-ol](https://img.chemicalbook.in/CAS/GIF/1174289-22-5.gif)
![4-[2-(1-AZEPANYL)ETHOXY]BENZYL CHLORIDE HCL](https://img.chemicalbook.in/CAS2/GIF/223251-25-0.gif)
![BenzeneMethanol, 4-[2-(hexahydro-1H-azepin-1-yl)ethoxy]- (hydrochloride)](https://img.chemicalbook.in/CAS/20150408/GIF/328933-65-9.gif)
![3-METHYL-5-(PHENYLMETHOXY)-2-[4-(PHENYLMETHOXY)PHENYL]-1H-INDOLE](https://img.chemicalbook.in/CAS/GIF/198479-63-9.gif)
![1-[4-(2-AZEPAN-1-YL-ETHOXY)-BENZYL]-5-BENZYLOXY-2-(4-BENZYLOXY-PHENYL)-3-METHYL-1H-INDOLE](https://img.chemicalbook.in/StructureFile/ChemBookStructure20/GIF/CB81009554.gif)
You may like
-
 198481-33-3 Bazedoxifene acetate 98%View Details
198481-33-3 Bazedoxifene acetate 98%View Details
198481-33-3 -
 198481-33-3 98%View Details
198481-33-3 98%View Details
198481-33-3 -
 Bazedoxifene acetate 98%View Details
Bazedoxifene acetate 98%View Details
198481-33-3 -
 Bazedoxifene acetate 96% CAS 198481-33-3View Details
Bazedoxifene acetate 96% CAS 198481-33-3View Details
198481-33-3 -
 Bazedoxifene acetate 98% (HPLC) CAS 198481-33-3View Details
Bazedoxifene acetate 98% (HPLC) CAS 198481-33-3View Details
198481-33-3 -
 Bazedoxifene Acetate API PowderView Details
Bazedoxifene Acetate API PowderView Details
198481-33-3 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8
