AVP-AEW541
Synonym(s):7-[cis-3-(1-Azetidinylmethyl)cyclobutyl]-5-[3-(phenylmethoxy)phenyl]-7H-pyrrolo[2,3-d]-pyrimidin-4-amine;AEW 541;AEW541;AEW-541;cis-7-(3-Aminomethyl-cyclobutyl)-5-(3-benzyloxy-phenyl)-7H-pyrrolo[2,3-d]-pyrimidin-4-ylamine
- CAS NO.:475489-16-8
- Empirical Formula: C27H29N5O
- Molecular Weight: 439.55
- MDL number: MFCD22741515
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-20 19:14:41
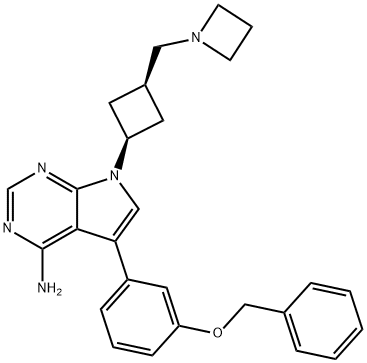
What is AVP-AEW541?
The Uses of AVP-AEW541
NVP-AEW541 is insulin-like growth factor I receptor (IGF-IR) inhibitor.
Biological Activity
nvp-aew541 is a novel, potent and selective inhibitor of igf-ir kinase with ic50 value of 0.086 μm [1].nvp-aew541 is a pyrrolo(2,3-d) pyrimidine derivative. it has been reported to abolish igf-i-induced igf-ir autophosphorylation and to block the igf-ir signaling pathway mainly in ecc-1 and uspc-1 cancer cells. also in these cell lines, nvp-aew541 has been shown to change the igf-i induced cell cycle and to lead apoptotic cell death as well as exhibit antiproliferative effects [2]. in addition, it is observed that nvp-aew541 can induce radiosensitization in pten wild-type cell lines [3].
Biochem/physiol Actions
NVP-AEW541 is a reversible, ATP-competitive, orally available insulin-like growth factor-I receptor (IGF-IR, IGF-1R) inhibitor that, despite its equipotency against recombinant kinase domains of insulin receptor (InsR) and IGF-IR in cell-free kinase assays (IC50 = 140 & 150 nM, respectively), exhibits high selectivity toward cellular autophosphorylation of endogenous full-length IGF-1R (IC50 = 86 nM) over InsR (IC50 = 2.3 μM), HER1, PDGFR, c-Kit, and Bcr-Abl p210 (IC50 >5 μM). AEW541 effectively inhibits IGF-IR-mediated signaling and cancer survival (IC50 = 105 nM/MCF-7 soft agar growth, 1.64 μM/NWT-21 proliferation) in cultures and tumor growth in vivo (20 to 50 mg/kg twice daily p.o.; NWT-21 fibrosarcoma & TC-71 musculoskeletal sarcoma xenografts in mice).
References
[1] carlos garc a-echeverr a, mark a. pearson, andreas marti, thomas meyer, juergen mestan, johann zimmermann, jiaping gao, josef brueggen, hans-georg capraro, robert cozens, dean b. evans, doriano fabbro, pascal furet, diana graus porta, janis liebetanz, georg martiny-baron, stephan ruetz, and francesco hofmann. in vivo antitumor activity of nvp-aew541—a novel, potent, and selective inhibitor of the igf-ir kinase. cancer cell.2004 mar (5):231-239.
[2] zohar attias-geva, itay bentov, ami fishman, haim werner, ilan bruchim. insulin-like growth factor-i receptor inhibition by speci c tyrosine kinase inhibitor nvp-aew541 in endometrioid and serous papillary endometrial cancer cell lines. gynecologic oncology. 2011 feb (121):383-389.
[3] sofie f. isebaert, johannes v. swinnen, william h. mcbride, and karin m. haustermans. insulin-like growth factor–type 1 receptor inhibitor nvp-aew541 enhances radiosensitivity of pten wild-type but not pten-deficient human prostate cancer cells. international journal of radiation oncology biology physics. 2011 (81):239-247.
Properties of AVP-AEW541
| Melting point: | 145℃ |
| Boiling point: | 669.0±55.0 °C(Predicted) |
| Density | 1.33 |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| pka | 9.59±0.10(Predicted) |
| form | powder |
| color | white to beige |
Safety information for AVP-AEW541
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for AVP-AEW541
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran
You may like
-
 NVP-AEW541 CAS 475489-16-8View Details
NVP-AEW541 CAS 475489-16-8View Details
475489-16-8 -
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 2856-63-5 99%View Details
2856-63-5 99%View Details
2856-63-5 -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5 -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8