Avobenzone
Synonym(s):1-(4-Methoxyphenyl)-3-(4-tert-butylphenyl)-1,3-propanedione;Butyl methoxydibenzoylmethane
- CAS NO.:70356-09-1
- Empirical Formula: C20H22O3
- Molecular Weight: 310.39
- MDL number: MFCD00210252
- EINECS: 274-581-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-27 14:23:08
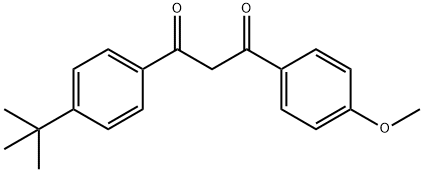
What is Avobenzone?
Toxicity
A minimum toxic dose has not been established. Significant toxicity is not expected
Background
Avobenzone is dibenzoyl methane derivative. It is oil soluble ingredient. Avobenzone has the ability to absorb ultraviolet light over wider range of wavelengths. It is included in many commercially available sunscreens which are used as wide spectrum sunscreens. Avobenzone is very sensitive to light, to increase its stability and duration of action, photostablizers are added in the sunscreen product. Avobenzone has an absorption maximum of 357 nm. Sunscreens containing avobenzone is indicated for providing protection from the sun. In addition to limiting the skin's exposure to the sun, using sunscreen agents may help reduce long-term sun damage such as premature aging of the skin and skin cancer.
Indications
Sun protection factor, added in the sunscreen products for its wide spectrum ultraviolet absorption properties.
Definition
ChEBI: Avobenzone is a member of dihydrochalcones.
General Description
Avobenzone is a dibenzoylmethane derivative for which the ground state is a mixture of the enol/ keto forms. Only the enol form is an effective UVA absorber. When irradiated with UVA light, it generates a triplet excited state in the keto form that can cause the avobenzone to degrade, as well as cause deep-level skin damage. Avobenzone can lose up to 36% of its potential for UV absorption after only 1 hour of exposure to sunlight, if not stabilized.
Synthesis
Avobenzone is produced synthetically by reacting 4-tert-butylbenzoic methyl ester (from 4-tert-butylbenzoic acid by esterification with methanol) with 4-methoxyacetophenone in toluene in the presence of sodium amide.
Properties of Avobenzone
| Melting point: | 81-84 °C |
| Boiling point: | 463.6±35.0 °C(Predicted) |
| Density | 1.079 |
| vapor pressure | 0Pa at 25℃ |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly, Heated, Sonicated) |
| form | neat |
| form | Solid |
| pka | 9.74±0.13(Predicted) |
| color | White to Pale Yellow |
| Water Solubility | 27μg/L at 20℃ |
| λmax | 356nm(EtOH)(lit.) |
| Merck | 14,888 |
| CAS DataBase Reference | 70356-09-1(CAS DataBase Reference) |
| EPA Substance Registry System | 1,3-Propanedione, 1-[4-(1,1-dimethylethyl)phenyl]-3-(4-methoxyphenyl)- (70356-09-1) |
Safety information for Avobenzone
| Signal word | Warning |
| Pictogram(s) |
 Environment GHS09 |
| GHS Hazard Statements |
H413:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P501:Dispose of contents/container to..… |
Computed Descriptors for Avobenzone
| InChIKey | GTIRDWBOUTYFQO-UHFFFAOYSA-N |
Avobenzone manufacturer
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran
You may like
-
 AVOBENZONE 99%View Details
AVOBENZONE 99%View Details -
 Avobenzone 98% CAS 70356-09-1View Details
Avobenzone 98% CAS 70356-09-1View Details
70356-09-1 -
 AvobenzoneView Details
AvobenzoneView Details
70356-09-1 -
 Avobenzone API PowderView Details
Avobenzone API PowderView Details
70356-09-1 -
 Avobenzone Powder ., 50 kgView Details
Avobenzone Powder ., 50 kgView Details
70356-09-1 -
 Avobenzone ChemicalView Details
Avobenzone ChemicalView Details
70356-09-1 -
 AvobenzoneView Details
AvobenzoneView Details
70356-09-1 -
 Organic Avobenzone Chemical, LooseView Details
Organic Avobenzone Chemical, LooseView Details
70356-09-1
