Ascorbic Acid
Synonym(s):Ascorbic Acid;Vitamin C;L(+)-Ascorbic Acid;LYAG;L -AA
- CAS NO.:50-81-7
- Empirical Formula: C6H8O6
- Molecular Weight: 176.12
- MDL number: MFCD03457784
- EINECS: 200-066-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-02-03 17:51:38
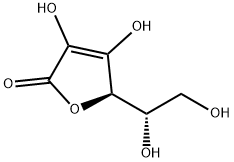
What is Ascorbic Acid?
Absorption
70% to 90%
Description
Ascorbic acid (vitamin C) is an antioxidant and water-soluble dietary supplement found in a variety of fruits and vegetables, such as citrus fruits, peppers, strawberries, and potatoes. Ascorbic acid plays an important role in maintaining health, helping to protect cells, maintain healthy skin, blood vessels, bones, and cartilage, and aid in wound healing. Vitamin C deficiency can lead to scurvy, so it is used to prevent and treat scurvy (a condition caused by a vitamin C deficiency that leads to fatigue, swollen gums, joint pain, and impaired wound healing). In the human body, L-ascorbic acid acts as an electron donor for eight different enzymes, including those involved in collagen hydroxylation, carnitine synthesis (which contributes to the production of adenosine triphosphate), norepinephrine synthesis, tyrosine metabolism, and peptide amidation. Its antioxidant activity may help reduce the risk of chronic diseases such as cancer, cardiovascular disease, and cataracts.
Chemical properties
Ascorbic acid is an odorless white crystal or crystalline powder with a sour taste. It is readily soluble in water, with aqueous solutions being strongly acidic. It is slightly soluble in alcohol and insoluble in ether, chloroform, benzene, petroleum ether, and oils. It is air-stable when dry but turns yellow with prolonged exposure to air. Its melting point is 190-192°C. It decomposes when melted. Its specific rotation is +20.5 to +21.5°. Ascorbic acid is a dibasic acid (pKa values of 4.1 and 11.8, respectively). It exists primarily as sodium and calcium salts. It is a strong reducing agent.
Originator
Ascorbic aci,Natur Product,France
History
Vitamin C is a general term for compounds having ascorbic acid activity, including ascorbic acid, dehydroascorbic acid, and its isomers.
The understanding of vitamin C has gone through a long and painful process. Although the relationship between scurvy and stored food is obvious, but the
treatments of this disease have been misguided. By 1601, British armed Captain
James Lancaster discovered the disease on the ship of the East India Company and
regarded the scurvy as “rot,” which could be made tissue alkaline.
At the early stage of the nineteenth century, the understanding and treatment of scurvy had developed to a right approach. The exposition of
scurvy etiology and metabolic theory took more than a century.
By the early stage of the twentieth century, inspired by the animal model of beriberi, researchers in the Christchurch Oslo University discovered one animal that
could suffer scurvy accidentally and then established a valuable scurvy animal
model. This experiment demonstrated that the extract isolated from lemon had antiscurvy activity. Until 1932, many research groups obtained the anti-scurvy crystal
from different plants and identified the crystal as ascorbic acid vitamin C. Next year,
the chemical structure of ascorbic acid was elucidated, and then its artificial synthesis was accomplished.
The Uses of Ascorbic Acid
Ascorbic acid is used as an antioxidant in foods, pharmaceuticals, and cosmetics. It combats free radical damage and protects against UVA damage, providing longer-lasting, broader protection when used in combination with traditional sunscreens. It also acts as a regulator of collagen biosynthesis, controlling intercellular colloidal substances (e.g., collagen), and, when formulated with a suitable carrier, can have a skin-whitening effect. There is evidence (albeit controversial) that it can penetrate the skin's layers and promote the healing of burned or damaged tissue. For this reason, it is found in burn creams and abrasion creams. It also has anti-aging, anti-inflammatory, antiviral, and immune system-boosting properties.
What are the applications of Application
L-Ascorbic acid, cell culture is a calcium channel protein inhibitor
Indications
Used to treat vitamin C deficiency, scurvy, delayed wound and bone healing, urine acidification, and in general as an antioxidant. It has also been suggested to be an effective antiviral agent.
Background
A six carbon compound related to glucose. It is found naturally in citrus fruits and many vegetables. Ascorbic acid is an essential nutrient in human diets, and necessary to maintain connective tissue and bone. Its biologically active form, vitamin C, functions as a reducing agent and coenzyme in several metabolic pathways. Vitamin C is considered an antioxidant.
Definition
ChEBI: L-ascorbic acid is the L-enantiomer of ascorbic acid and conjugate acid of L-ascorbate. It has a role as a coenzyme, a flour treatment agent, a food antioxidant, a plant metabolite, a cofactor, a skin lightening agent and a geroprotector. It is an ascorbic acid and a vitamin C. It is a conjugate acid of a L-ascorbate. It is an enantiomer of a D-ascorbic acid.
Production Methods
Ascorbic acid is synthesized using the Reichstein process, which involves fermentation followed by chemical synthesis. The first step involves the reduction of D-glucose to D-sorbitol at high temperature. D-sorbitol is then converted to L-sorbose through bacterial fermentation. L-sorbose then reacts with acetone in the presence of concentrated sulfuric acid to produce diacetone-L-sorbose, which is then oxidized with chlorine and sodium hydroxide to produce diacetone-ketogulonic acid (DAKS). DAKS is then esterified with an acidic catalyst and organic matter to produce methyl gulonic acid. Methyl gulonic acid is then heated and reacted with alcohol to produce crude ascorbic acid, which is then recrystallized to improve its purity. A subsequent two-stage fermentation process was developed, utilizing a second stage of fermentation of L-sorbose to produce an intermediate distinct from DAKS, called KGA (2-keto-L-gulonic acid), which is then converted to ascorbic acid.
Indications
Vitamin C (ascorbic acid) is essential for the maintenance of the ground substance that binds cells together and for the formation and maintenance of collagen.The exact biochemical role it plays in these functions is not known, but it may be related to its ability to act as an oxidation–reduction system.
Manufacturing Process
D-Glucose was reduced to the D-sorbitol with a hydrogen over Ni Raney, then it was turned into the L-sorbose with the acetobacter suboxydans and the hydroxyl groups of L-sorbose were protected with acetone treatment yielded the diaceton-L-sorbose. Subsequent treatment with NaOCl/Raney Ni produced di-O-isopropylidene-2-oxo-L-gulonic acid. Partial hydrolysis with aqueous HCl gave deprotected 2-oxo-L-gulonic acid, which yielded ascorbinic acid by heating with HCl.
brand name
Ascorbin (Marion Merrell Dow).
Therapeutic Function
Vitamin
General Description
White to very pale yellow crystalline powder with a pleasant sharp acidic taste. Almost odorless.
Air & Water Reactions
May be sensitive to prolonged exposure to air and light. Sensitive to moisture. Soluble in water. Aqueous solutions are oxidized by air in a reaction that is accelerated by alkalis, iron and copper. The rate depends on the pH and on oxygen concentration. Also subject to degradation under anaerobic conditions.
Reactivity Profile
L(+)-Ascorbic acid is a lactone. Reacts as a relatively strong reducing agent and decolorizes many dyes. Forms stable metal salts. Incompatible with oxidizers, dyes, alkalis, iron and copper. Also incompatible with ferric salts and salts of heavy metals, particularly copper, zinc and manganese .
Fire Hazard
Flash point data for L(+)-Ascorbic acid are not available; however, L(+)-Ascorbic acid is probably combustible.
Biochem/physiol Actions
L-ascorbic acid mainly exhibits antioxidant properties. It protects plants from oxidative stress and mammals from diseases associated with oxidative stress. L-ascorbic acid mainly protects from hydroxyl radicals, superoxide and singlet oxygen. In addition, it also reduces the membrane-linked antioxidant α-tocopherol (oxidised form). L-Ascorbic acid enhances endothelium-dependent vasodilation in various disorders, including diabetes, coronary artery disease, hypertension and chronic heart failure.
Pharmacokinetics
Ascorbic Acid (vitamin C) is a water-soluble vitamin indicated for the prevention and treatment of scurvy, as ascorbic acid deficiency results in scurvy. Collagenous structures are primarily affected, and lesions develop in bones and blood vessels. Administration of ascorbic acid completely reverses the symptoms of ascorbic acid deficiency.
Pharmacology
Vitamin C is considered as a classical enzyme cofactor or antioxidant but also as a transition material in metal ion reaction. And all of these functions of vitamin C are related to the property of antioxidation.
Clinical Use
Vitamin C is indicated for the treatment and prevention of known or suspect deficiency. Although scurvy occurs infrequently, it is seen in the elderly, infants, alcoholics, and drug users.Ascorbate can also be used to enhance absorption of dietary nonheme iron or iron supplements. The deficiency disease associated with a lack of ascorbic acid is called scurvy. Early symptoms include malaise and follicular hyperkeratosis. Capillary fragility results in hemorrhages, particularly of the gums. Abnormal bone and tooth development can occur in growing children.The body’s requirement for vitamin C increases during periods of stress, such as pregnancy and lactation.
Side Effects
Megavitamin intake of vitamin C may result in diarrhea due to intestinal irritation. Since ascorbic acid is partially metabolized and excreted as oxalate, renal oxalate stones may form in some patients.
Safety Profile
Moderately toxic by ingestion and intravenous routes. Human systemic effects by intravenous route: blood, changes in tubules (including acute renal failure, acute tubular necrosis). An experimental teratogen. Other experimental reproductive effects. Mutation data reported. When heated to decomposition it emits acrid smoke and irritating fumes.
Toxicology
L-Ascorbic acid, or vitamin C, is widely present in plants. The structures of ascorbic acid and dehydroascorbic acid are shown in Figure 10.5. Vitamin C is not only an important nutrient but is also used as an antioxidant in various foods. However, it is not soluble in fat and is unstable under basic conditions. Vitamin C reduces cadmium toxicity and excess doses prolong the retention time of an organic mercury compound in a biological system. Overdoses of vitamin C (106 g) induce perspiration, nervous tension, and lowered pulse rate. WHO recommends that daily intake be less than 0.15 mg/kg. Toxicity due to ascorbic acid has not been reported. Although repeated intravenous injections of 80 mg dehydroascorbic acid was reported to be diabetogenic in rats, oral consumption of 1.5 g/day of ascorbic acid for six weeks had no effect on glucose tolerance or glycosuria in 12 normal adult males and produced no change in blood glucose concentrations in 80 diabetics after five days. The same report noted that a 100-mg intravenous dose of dehydroascorbic acid given daily for prolonged periods produced no signs of diabetes. Ascorbic acid is readily oxidized to dehydroascorbic acid, which is reduced by glutathione in blood.
Metabolism
Hepatic. Ascorbic acid is reversibly oxidised (by removal of the hydrogen from the enediol group of ascorbic acid) to dehydroascorbic acid. The two forms found in body fluids are physiologically active. Some ascorbic acid is metabolized to inactive compounds including ascorbic acid-2-sulfate and oxalic acid.
Purification Methods
Crystallise it from MeOH/Et2O/pet ether [Herbert et al. J Chem Soc 1270 1933]. [Beilstein 18/5 V 26.]
Toxicity evaluation
Metabolism of ascorbic acid can lead to deposition of oxalate crystals in kidney tissue. Reduction of carcinogenic Cr(VI) by ascorbic acid generates ascorbate–Cr(III)–DNA cross-links that have been linked to mutagenicity and the formation of DNA lesions. Uranyl acetate–ascorbate has also been shown to nick plasmid DNA.
Properties of Ascorbic Acid
| Melting point: | 190-194 °C (dec.) |
| Boiling point: | 227.71°C (rough estimate) |
| alpha | 20.5 º (c=10,H2O) |
| Density | 1,65 g/cm3 |
| refractive index | 21 ° (C=10, H2O) |
| FEMA | 2109 | ASCORBIC ACID |
| storage temp. | Store at +5°C to +30°C. |
| solubility | H2O: 50 mg/mL at 20 °C, clear, nearly colorless |
| form | powder |
| appearance | white to pale yellow crystals or powder |
| pka | 4.04, 11.7(at 25℃) |
| color | white to slightly yellow |
| Odor | Odorless |
| PH | 3.59(1 mM solution);3.04(10 mM solution);2.53(100 mM solution); |
| PH Range | 1 - 2.5 |
| optical activity | [α]25/D 19.0 to 23.0°, c = 10% in H2O |
| Water Solubility | 333 g/L (20 ºC) |
| Merck | 14,830 |
| BRN | 84272 |
| Stability: | Stable. May be weakly light or air sensitive. Incompatible with oxidizing agents, alkalies, iron, copper. |
| CAS DataBase Reference | 50-81-7(CAS DataBase Reference) |
| NIST Chemistry Reference | L-Ascorbic acid(50-81-7) |
| EPA Substance Registry System | Ascorbic acid (50-81-7) |
Safety information for Ascorbic Acid
| Signal word | Warning |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H226:Flammable liquids H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P370+P378:In case of fire: Use … for extinction. |
Computed Descriptors for Ascorbic Acid
| InChIKey | CIWBSHSKHKDKBQ-JLAZNSOCSA-N |
Ascorbic Acid manufacturer
New Products
N,O-Dimethylhydroxylamine hydrochloride DL-beta-(3-Bromophenyl)alanine N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid (R)-1-Benzyl-3-pyrrolidinecarbonitrile Bupropian related compound F Lantanoprost rc B Clidinium Bromide Impurity Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity N-Nitroso hydroxy Cetrizine EP Impurity-A 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 2-Chloro Benzylcyanide 3-chlorobenzyl cyanide 3,4 Diethoxy Benzylcyanide 3,4 Dimethoxy Benzylcyanide 3-Hydroxypropionitrile 4-Bromo BenzylcyanideRelated products of tetrahydrofuran
You may like
-
 Ascorbic Acid 98%View Details
Ascorbic Acid 98%View Details -
 Vitamin C 99%View Details
Vitamin C 99%View Details -
 Ascorbic Acid 99%View Details
Ascorbic Acid 99%View Details -
 L-(+)-Ascorbic acid CAS 50-81-7View Details
L-(+)-Ascorbic acid CAS 50-81-7View Details
50-81-7 -
 L-(+)-Ascorbic acid CAS 50-81-7View Details
L-(+)-Ascorbic acid CAS 50-81-7View Details
50-81-7 -
 L-(+)-Ascorbic acid CAS 50-81-7View Details
L-(+)-Ascorbic acid CAS 50-81-7View Details
50-81-7 -
 Ascorbic Acid CASView Details
Ascorbic Acid CASView Details -
 L-(+)-Ascorbic acid CAS 50-81-7View Details
L-(+)-Ascorbic acid CAS 50-81-7View Details
50-81-7
