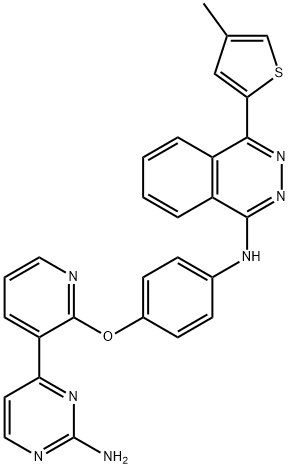
Apatinib
- CAS NO.:811803-05-1
- Empirical Formula: C24H23N5O
- Molecular Weight: 397.47
- MDL number: MFCD22420814
- EINECS: 1592732-453-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:25:01
What is Apatinib?
Description
Apatinib mesylate, discovered by Advenchen Laboratories (United States of America, USA) and co-developed by Jiangsu Hengrui Medicine Co. Ltd (China), was approved by the Chinese Food and Drug Administration (CFDA) in October 2014 for the treatment of metastatic gastric carcinoma. Apatinib mesylate is an oral tyrosine kinase inhibitor that selectively inhibits the vascular endothelial growth factor receptor 2 (VEGFR2), which prevents new blood vessel formation selectively in tumor tissue. Apatinib has shown inhibition of the VEGF signaling pathway with an IC50 value of 1 nM for VEGFR-2 in in vitro enzyme experiments. A multicenter phase II study of apatinib is underway with patients in non-triple-negative metastatic breast cancer trials. Non-clinical studies concluded that apatinib may reverse the ATP-binding cassette subfamily B member 1 and subfamily G member 2 (ABCB1- and ABCG2, respectively)-mediated multidrug resistance which allows cancer cells to circumvent certain conventional antineoplastic drugs, suggesting that apatinib could be effective as a combination therapy.
Description
Apatinib is a tyrosine kinase inhibitor that potently suppresses the kinase activity of vascular endothelial growth factor 2 (VEGFR2; IC50 = 1 nM). It is less effective against c-kit (IC50 = 429 nM), Ret (IC50 = 13 nM), and c-src (IC50 = 53 nM) and does not inhibit EGFR, Her-2, or FGFR1 (IC50s = >10 μM). Apatinib has been shown to inhibit the proliferation, migration, and tube formation of human umbilical vein endothelial cells stimulated by fetal bovine serum and, either alone, or in combination with chemotherapeutic agents, prevented the growth of several established human tumor xenograft models.
The Uses of Apatinib
Apatinib (YN968D1) is a small-molecule selective multitargeted tyrosine kinase inhibitor with an IC50 of 2.43 nM for the inhibition of VEGFR2.
Synthesis
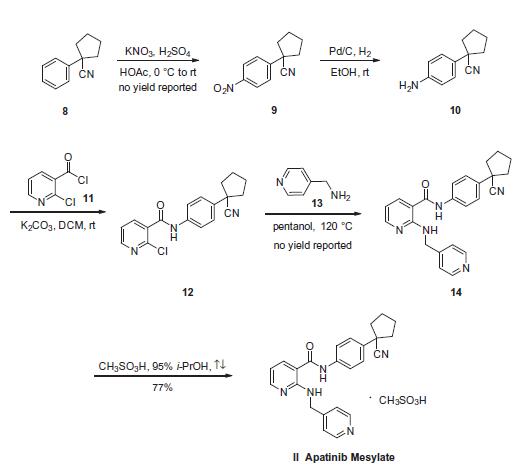
The synthesis started with commercially available 1-phenyl cyclopentane carbonitrile (8), which was nitrated to provide nitrobenzene 9. Subsequent reduction of 9 gave aniline 10, which was coupled with 2-chloronicotinoyl chloride (11) to afford aryl amide 12. Subjection of the 2-pyridyl chloride within 12 to pyridin-4-ylmethanamine (13) in hot pentanol gave 14. The preparation of apatinib 14 from starting material 8 were reported on gram or milligram reaction scale with no yield. 170 g of 14 was mixed with methylsulfonic acid in 95% isopropanol¨CH2O solution to give 161.5 g of apatinib mesylate (II) in 77% yield.
Properties of Apatinib
| Boiling point: | 578.2±50.0 °C(Predicted) |
| Density | 1.27 |
| storage temp. | Store at -20°C |
| solubility | >49.4mg/ml in DMSO |
| form | Powder |
| pka | 11.93±0.70(Predicted) |
Safety information for Apatinib
Computed Descriptors for Apatinib
New Products
Cyclopropane-1,1-dicarboxylic acid Cyclopentane-1,2-dione 2-Bromo-5-iodopyridine 4-isothiocyanato-2-(trifluoroMethyl)benzonitrile Amino-4-methoxyben-zeneacetic acid 3-Aminophenylacetic acid 1-AMino-cyclobutaneMethanol HCl (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid Cefuroxime EP Impurity-A N-Nitroso hydroxy Cetrizine EP Impurity-A Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity Lantanoprost rc B Clidinium Bromide Impurity 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro Benzylcyanide 4-Bromo Benzylcyanide 5-azidovalericacid 4-bromo-1H-pyrazole-3-carboxylic acid Isopropyl amine Hydrochloride tert-butyl 4-(6-(8-cyclopentyl-5-methyl-7-oxo-6-vinyl-7,8-dihydropyrido[2,3-d]pyrimidin-2-ylamino)pyridin-3-yl)piperazine-1-carboxylateRelated products of tetrahydrofuran
You may like
-
 Apatinib 98% (HPLC) CAS 811803-05-1View Details
Apatinib 98% (HPLC) CAS 811803-05-1View Details
811803-05-1 -
 4-cyano-2,2-dimethylbutanoic acid 6939-69-1 98+View Details
4-cyano-2,2-dimethylbutanoic acid 6939-69-1 98+View Details
6939-69-1 -
 1-(cyanomethyl)-1-cyclopentanecarbonitrile 98+View Details
1-(cyanomethyl)-1-cyclopentanecarbonitrile 98+View Details
1539-03-3 -
 tert-butyl(R)-4-(methyl(1-(pyrrolidin-3-yl)cyclopropyl)amino)piperidine-1-carboxylate 922718-06-7 98+View Details
tert-butyl(R)-4-(methyl(1-(pyrrolidin-3-yl)cyclopropyl)amino)piperidine-1-carboxylate 922718-06-7 98+View Details
922718-06-7 -
 39773-47-2 (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 98+View Details
39773-47-2 (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 98+View Details
39773-47-2 -
 1392213-15-8 98+View Details
1392213-15-8 98+View Details
1392213-15-8 -
 39773-47-2 (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 98+View Details
39773-47-2 (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 98+View Details
39773-47-2 -
 1392213-15-8 98+View Details
1392213-15-8 98+View Details
1392213-15-8