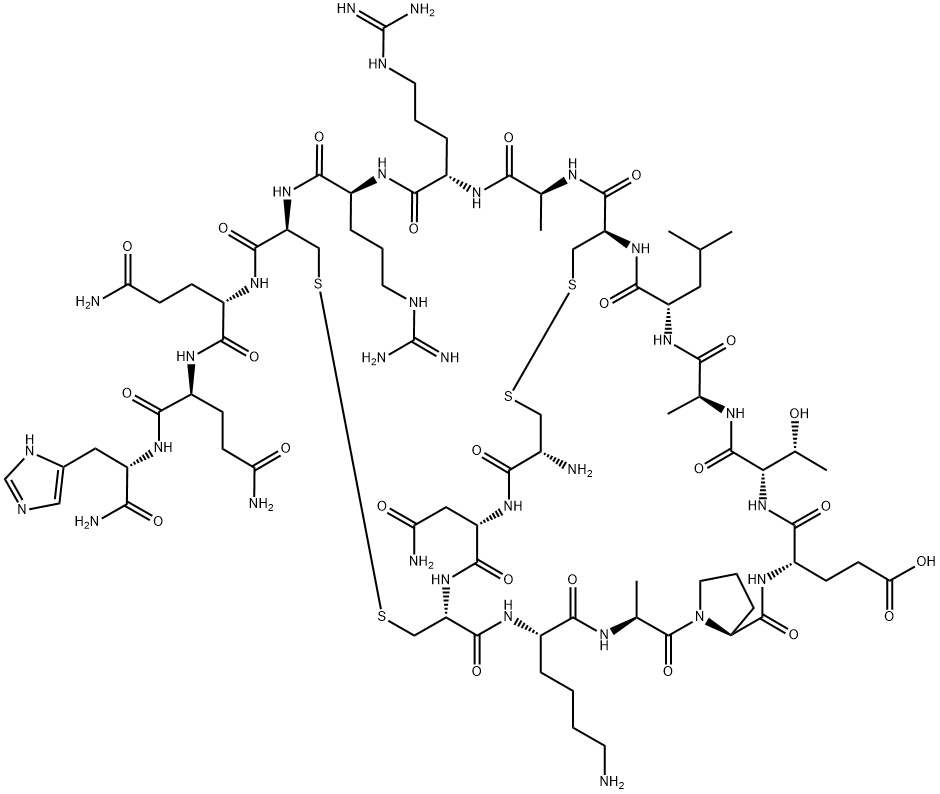
APAMIN
Synonym(s):Apamin, Bee Venom - CAS 24345-16-2 - Calbiochem;CNCKAPETALCARRCQQH-NH₂
- CAS NO.:24345-16-2
- Empirical Formula: C79H131N31O24S4
- Molecular Weight: 2027.34
- MDL number: MFCD00167944
- EINECS: 246-182-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:19:40
What is APAMIN?
Chemical properties
Small, highly potent, highly basic polypeptide.
The Uses of APAMIN
Apamin has been used:
- As a selective inhibitor of small conductance (SKCa) channels in HEK cells.
- To inhibit endothelium-derived relaxing factor (EDHF) mediated responses.
- To block small-conductance Ca2-activated K current (ISK) in electrophysiological studies in hyperstriatum ventrale, pars caudalis (HVc) neurons.
What are the applications of Application
Apamin is a potent and highly selective inhibitor of the low conductance Ca2+-activated K+ channel
Definition
Occurs in the venom of the honey bee, Apis mellifera; molecular weight of 2027.38.
General Description
Apamin is a bee venom component and is strongly basic in nature. It has 18 amino acid residues and two disulphide bonds.
Hazard
Central nervous system poison able to cross the blood–brain barrier; neurotoxic; poison.
Biochem/physiol Actions
Apamin is a neurotoxin which can pass the blood-brain barrier. In human, it might cause peripheral nerve dysfunction, mainly seen after bee stings.
Safety Profile
Poison by intravenous, parenteral,intracerebral, and intraperitoneal routes. When heated todecomposition it emits toxic fumes of SOx and NOx.
Enzyme inhibitor
This bee venom octadecapeptide toxin (FW = 2027.34 g/mol; CAS 24345- 16-2; NCBI Reference Sequence: NP_001011612.1) potently blocks the small-conductance Ca 2+ -activated potassium ion (or SK) channels hSK1 as well as rSK2, with IC50 values of 3.3 nM and 83 pM. It shows greater effectiveness at the SK2 channel (IC50 = 0.06-0.4 nM) than SK1 (IC50 = 1- 12 nM). SK3 (IC50 = 1-13 nM), and SK4 (IC50 = 1 μM) channels, and is active against channels within neurons, vascular endothelium, bladder smooth muscle, and certain cancers. Apamin does not inhibit human cardiac Na + current, L-type Ca 2+ current or other major K+ currents. Structurally, apamin forms a stable structure, consisting of a C-terminal a- helix and two reverse turns, that is stabilized by two disulfide bonds connecting Cys-1 to Cys-11 and Cys-3 to Cys-15. A minor constituent of venom of the bee (Apis mellifera), apamin amounts to only 2-3% (w/w) of its dry venom. The smallest known neurotoxic polypeptide, apamin is derived by proteolytic processing of the 48-residue pre-pro-protein. The precursors of the bee venom constituents apamin and MCD peptide are encoded by two genes in tandem which share the same 3'-exon.
Storage
-20°C (desiccate)
Properties of APAMIN
| Boiling point: | 847.17°C (rough estimate) |
| Density | 1.63 |
| refractive index | 1.5530 (estimate) |
| storage temp. | -20°C |
| solubility | 0.05 M acetic acid: 5 mg/mL, clear, colorless to faintly yellow |
| form | White solid with dark tan |
| color | White to off-white |
| Water Solubility | Soluble to 1 mg/ml in water |
| Merck | 13,732 |
Safety information for APAMIN
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H334:Sensitisation, respiratory |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P342+P311:IF experiencing respiratory symptoms: call a POISON CENTER or doctor/physician. |
Computed Descriptors for APAMIN
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile 3,4 Dimethoxy Benzylcyanide valeronitrile 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran

You may like
-
 Apamin CAS 24345-16-2View Details
Apamin CAS 24345-16-2View Details
24345-16-2 -
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5 -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 5-azidovalericacidView Details
5-azidovalericacidView Details
79583-98-5
