5,7-Dichloro-8-hydroxyquinaldine
Synonym(s):5,7-Dichloro-2-methyl-8-quinolinol;5,7-Dichloro-8-hydroxyquinaldine;5,7-Dichloro-8-quinaldinol;BCM
- CAS NO.:72-80-0
- Empirical Formula: C10H7Cl2NO
- Molecular Weight: 228.07
- MDL number: MFCD00023984
- EINECS: 200-789-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-21 16:49:52
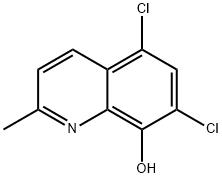
What is 5,7-Dichloro-8-hydroxyquinaldine?
Absorption
There is a high degree of variability in the extent of absorption of topically applied chorquinaldol preparations. It is reported to be between 4.2 and 23.5% of the applied dose. This is quite low compared to orally administered preparations which displayed 67.6% absorption in the same study.
Toxicity
Oral LD50 (rat) - 660mg/kg
Chemical properties
yellowish to beige-brown powder
Originator
Sterosan ,Geigy ,US ,1954
The Uses of 5,7-Dichloro-8-hydroxyquinaldine
antiinfectant, antifungal
The Uses of 5,7-Dichloro-8-hydroxyquinaldine
Chlorquinaldol is a fungistat and antibacterial agent in topical pharmaceutical preparations (5,7-dichloro-2-methyl-8-quinolinol, Sterosan).
The Uses of 5,7-Dichloro-8-hydroxyquinaldine
5,7-Dichloro-8-hydroxy-2-methylquinoline is an anti-infective agent.
Indications
Chlorquinaldol was used historically as a topical antiseptic agent for skin infections. It maintains use in European countries as a combination vaginal tablet with promestriene for use in the treatment of vaginal infections.
What are the applications of Application
5,7-Dichloro-8-hydroxy-2-methylquinoline is an anti-infective agent
Background
Chlorquinaldol was used historically as a topical antiseptic under the trade name Sterosan. It was marketed in the 1950s as an iodine-free alternative which was also unrelated to sulfa drugs or hormones. Chlorquinaldol is currently approved by the European Medicines Agency as a combination tablet with promestriene for the treatment of bacterial vaginosis.
Definition
ChEBI: A monohydroxyquinoline that is quinolin-8-ol which is substituted by a methyl group at position 2 and by chlorine at positions 5 and 7. An antifungal and antibacterial, it was formerly used for topical treatment of skin conditions and vaginal infections.
Manufacturing Process
11.1 parts of 8-hydroxy-quinaldine are dissolved in 140 parts of formic acid.
Chlorine is introduced into this solution under cooling, until the increase in
weight corresponds to the required quantity of chlorine and a test of the
chlorination mixtures gives no more dyestuff formation with diazo-benzene in
an acetic acid solution
When the chlorination is complete, the reaction mixture is poured into 1,000
parts of water and treated with a dilute sodium bisulfite solution, until no
more reaction may be observed with starch potassium iodide paper. Thereby
the 5,7-dichloro-8-hydroxy-quinaldine separates out in form of a weakly
yellowish colored precipitate. The same is filtered off and thoroughly washed
with water.After drying, 15 parts of 5,7-dichloro-8-hydroxy-quinaldine melting at 111°C
to 112°C are obtained. When recrystallized from alcohol, the product is
obtained in voluminous, slightly yellowish needles having the melting point of
111.5°C to 112°C.
Therapeutic Function
Antibacterial
Pharmacokinetics
Chlorquinaldol is bacteriocidal in both gram positive and gram negative bacteria. It is more effective in targeting gram positive bacteria, particularly staphylococci.
Metabolism
98% of drug is converted to the sulfate form and renally excreted.
Purification Methods
Crystallise it from EtOH. [Beilstein 21/3 V 346.]
Properties of 5,7-Dichloro-8-hydroxyquinaldine
| Melting point: | 108-112 °C (dec.)(lit.) |
| Boiling point: | 350.7±37.0 °C(Predicted) |
| Density | 1.3126 (rough estimate) |
| refractive index | 1.5500 (estimate) |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | DMSO, Methaol (Slightly) |
| form | Solid |
| pka | 2.47±0.30(Predicted) |
| color | Dark Beige to Very Dark Beige |
| Water Solubility | Insoluble |
| Merck | 13,2209 |
| BRN | 156683 |
| CAS DataBase Reference | 72-80-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Chlorquinaldol(72-80-0) |
Safety information for 5,7-Dichloro-8-hydroxyquinaldine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
Computed Descriptors for 5,7-Dichloro-8-hydroxyquinaldine
5,7-Dichloro-8-hydroxyquinaldine manufacturer
SAKEM LLP
New Products
DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateRelated products of tetrahydrofuran



You may like
-
 72-80-0 8-HYDROXY-2-METHYL QUINOLINE 98%View Details
72-80-0 8-HYDROXY-2-METHYL QUINOLINE 98%View Details
72-80-0 -
 5,7-Dichloro-8-hydroxy-2-methylquinoline CAS 72-80-0View Details
5,7-Dichloro-8-hydroxy-2-methylquinoline CAS 72-80-0View Details
72-80-0 -
 Chlorquinaldol >98% CAS 72-80-0View Details
Chlorquinaldol >98% CAS 72-80-0View Details
72-80-0 -
 8-Hydroxy-2-Methyl Quinoline 72-80-0 98%View Details
8-Hydroxy-2-Methyl Quinoline 72-80-0 98%View Details
72-80-0 -
 674783-97-2 98+View Details
674783-97-2 98+View Details
674783-97-2 -
 H-D-TRP(FOR)-OH HCL 98+View Details
H-D-TRP(FOR)-OH HCL 98+View Details
367453-01-8 -
 1428243-26-8 98+View Details
1428243-26-8 98+View Details
1428243-26-8 -
 1-aminocyclopentane carbonitrile, HCl 98+View Details
1-aminocyclopentane carbonitrile, HCl 98+View Details
16195-83-8
