5-Methyl-2-phenyl-1,2-dihydropyrazol-3-one
Synonym(s):Edaravone;1-Phenyl-3-methyl-5-pyrazolone;3-Methyl-1-phenyl-2-pyrazolin-5-one;3-Methyl-1-phenyl-2-pyrazoline-5-one;5-Methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one
- CAS NO.:89-25-8
- Empirical Formula: C10H10N2O
- Molecular Weight: 174.2
- MDL number: MFCD00003138
- EINECS: 201-891-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-30 17:41:12
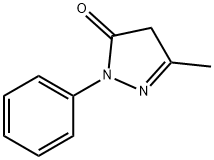
What is 5-Methyl-2-phenyl-1,2-dihydropyrazol-3-one?
Absorption
One study investigated the absorption of edaravone in healthy adults, who either received a single oral (105 mg/mL) or intravenous (60 mg/60 min) dose. The mean Cmax (CV%) and Tmax were 1656 (44.3) ng/mL and 0.5 hours, respectively, following oral administration. The absolute oral bioavailability is about 57% because of first-pass metabolism.
The mean Cmax (CV%) and Tmax were 1253 (18.3) ng/mL and one hour, respectively, following intravenous administration. When intravenously administered, the maximum plasma concentration (Cmax) of edaravone was reached by the end of infusion.
The Cmax and area under the concentration-time curve (AUC) of edaravone increases more than dose-proportional over the dose range of 30 to 300 mg. Edaravone does not accumulate in plasma with once-daily or multiple-dose administration. The Cmax and AUC decreased when the oral suspension formulation of edaravone was administered with a high-fat meal.
Toxicity
Oral and intravenous LD50 in rats are 1915 mg/kg and 631 mg/kg, respectively. There is limited information on the acute toxicity of edaravone.
Description
Edaravone also known as 1-phenyl-3-methyl-5-pyrazolone or 3-Methyl-1-phenylpyrazol-5-one is an antioxidant with free radical scavenging activity that improves neurological recovery after acute cerebral infarction. It is also a lipophilic agent that readily enters brain tissue and is able to reduce oedema after cerebral ischaemia by blocking the arachidonic acid cascade reaction that triggers peroxisomal neurodegeneration. It also plays an effective role in the reperfusion process following ischaemic injury.
Description
MCI-
Chemical properties
Off white to light yellow powder
Originator
Mitsubishi Pharma (Japan)
The Uses of 5-Methyl-2-phenyl-1,2-dihydropyrazol-3-one
Edaravone inhibits the disease activity in rheumatoid arthritis.
The Uses of 5-Methyl-2-phenyl-1,2-dihydropyrazol-3-one
antioxidant, lipoxygenase inhibitor
The Uses of 5-Methyl-2-phenyl-1,2-dihydropyrazol-3-one
3-Methyl-1-phenyl-2-pyrazolin-5-one used as reagent for detection of reducing carbohydrates by ESI/MALDI -MS.
Indications
Edaravone is indicated for the treatment of amyotrophic lateral sclerosis (ALS) in the US and Canada. It is also indicated to treat acute ischemic stroke in Japan.
Background
Edaravone is a free radical scavenger and neuroprotective agent with antioxidant properties. It has three tautomers. Edaravone works to scavenge reactive oxygen species, which have been implicated in neurological disorders, such as amyotrophic lateral sclerosis (ALS) and cerebral ischemia.
The intravenous formulation of edaravone was first approved in Japan in 2001 for the treatment of acute ischemic stroke. It was later approved for the treatment of amyotrophic lateral sclerosis (ALS) in Japan and South Korea in 2015, followed by the FDA approval in May 2017 and Health Canada approval in October 2018. The oral suspension formulation of edaravone was approved by the FDA in May 2022 and by Health Canada in November 2022.
Edaravone was initially granted orphan designation by the European Medicines Agency on June 19, 2015 and was under regulatory review in Europe. However, the drug manufacturer, Mitsubishi Tanabe Pharma, withdrew the Marketing Authorization Application (MAA) for edaravone from the European market on May 24, 2019, in response to the request made by the Committee for Medicinal Products for Human Use (CHMP) for a long-term study demonstrating the long-term efficacy and safety of edaravone. Edaravone was also investigated in other disorders, such as Alzheimer's disease, neuropathic pain, and ischemia-induced nerve injury.
What are the applications of Application
MCI-186 is a free radical scavenger that enhances NGF protein levels via the JNK pathway
Definition
ChEBI: A pyrazolone that is 2,4-dihydro-3H-pyrazol-3-one which is substituted at positions 2 and 5 by phenyl and methyl groups, respectively.
brand name
Radicut
General Description
A free radical scavenger and antioxidant that reduces post-ischemic brain injury. Inhibits iron-dependent peroxidation in rat brain homogenates (IC50 = 15 μM). Inhibits mitochondrial permeability transition pore.
Hazard
Toxic by ingestion.
Flammability and Explosibility
Not classified
Mechanism of action
Edaravon acts as a free radical scavenger and reduces oxidative stress and reactive oxygen species (ROS) production. Since oxidative stress is associated with the pathophysiology of ALS and cerebral ischaemia, inhibition of lipid peroxidation, suppression of lipid peroxide-induced endothelial cell damage, and scavenging of free radicals may have neuroprotective effects. In addition, Edaravon may have anti-inflammatory properties as it inhibits neutrophil activation and inhibits the expression of inducible nitric oxide synthase (iNOS) and neuronal nitric oxide synthase (nNOS) in animal models. It has also been shown to ameliorate ROS-induced inflammatory oxidative stress after ischaemic cerebral reperfusion.
Biological Activity
A radical scavenger and antioxidant which is able to protect against the effects of ischemia, probably by inhibiting the lipoxygenase system. Protects against MPTP-induced neurotoxicity.
Biochem/physiol Actions
Product does not compete with ATP.
Pharmacokinetics
Edaravone works to delay the disease progression of neurological disorders such as ischemic stroke and ALS by limiting the extent of neuronal damage or death.
Safety Profile
Moderately toxic by ingestion andintraperitoneal routes. An eye irritant. When heated todecomposition it emits toxic fumes of NOx.
Metabolism
The metabolites of edaravone have not been fully characterized. Edaravone is metabolized to a sulfate conjugate and a glucuronide conjugate, which are not pharmacologically active. The glucuronide conjugation of edaravone involves multiple uridine diphosphate glucuronosyltransferase (UGT) isoforms (UGT1A1, UGT1A6, UGT1A7, UGT1A8, UGT1A9, UGT1A10, UGT2B7, and UGT2B17). In human plasma, edaravone is mainly detected as the sulfate conjugate, which is presumed to be formed by sulfotransferases. Oral edaravone results in 1.3- and 1.7-fold higher exposures for both sulfate and glucuronide metabolites, respectively, when compared to intravenously-administered edaravone because of first-pass metabolism.
Side Effects
Common side effects of Edaravon include dizziness, headache, cough, itchy skin, dry and cracked skin, peeling of the skin, fast heartbeat, weakness, unusual bruising, bluish discolouration of the lips, nails or skin, chest pain or tightness in the chest, changes in walking and balance, hives, rash or swelling. A few patients may have symptoms of urinary sugar. Contact your doctor promptly if you have any serious adverse reactions.
Storage
Store at RT
Purification Methods
Crystallise the pyrazolone from hot H2O, EtOH or EtOH/water (1:1). It complexes with metals. [Veibel et al. Acta Chim Scand 6 1066 1952, Beilstein 24 II 9, 24 III/IV 71.]
References
1) Watanabe et al. (1994), Protective effects of MCI-186 on cerebral ischemia:possible involvement of free radical scavenging and antioxidant actions; J. Pharmacol. Exp. Ther., 268 1597 2) Yoshida et al. (2006) Neuroprotective effects of edaravone:a novel free radical scavenger in cerebrovascular injury; CNS Drug Reviews, 12 9 3) Kawasaki et al. (2007) Edaravone (3-Methyl-1-phenyl-2-pyrazolin-5-one), a Radical Scavenger, Prevents 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Neurotoxicity in the Substantia Nigra but Not the Striatum; J. Pharmacol. Exp. Ther., 322 274
Properties of 5-Methyl-2-phenyl-1,2-dihydropyrazol-3-one
| Melting point: | 126-128 °C(lit.) |
| Boiling point: | 287 °C265 mm Hg(lit.) |
| Density | 1,12 g/cm3 |
| vapor pressure | 0.016Pa at 20℃ |
| refractive index | 1.6300 (estimate) |
| Flash point: | 191°C/17mm |
| storage temp. | 2-8°C |
| solubility | 3.30g/l |
| form | Crystalline Powder |
| pka | 2.73±0.50(Predicted) |
| color | Yellow to beige |
| PH | 4.0-4.4 (H2O, 20℃)(saturated aqueous solution) |
| Water Solubility | 3 g/L (20 ºC) |
| Merck | 14,6713 |
| BRN | 609575 |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 3 months. |
| CAS DataBase Reference | 89-25-8(CAS DataBase Reference) |
| NIST Chemistry Reference | 3H-Pyrazol-3-one, 2,4-dihydro-5-methyl-2-phenyl-(89-25-8) |
| EPA Substance Registry System | 1-Phenyl-3-methyl-5-pyrazolone (89-25-8) |
Safety information for 5-Methyl-2-phenyl-1,2-dihydropyrazol-3-one
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for 5-Methyl-2-phenyl-1,2-dihydropyrazol-3-one
| InChIKey | QELUYTUMUWHWMC-UHFFFAOYSA-N |
5-Methyl-2-phenyl-1,2-dihydropyrazol-3-one manufacturer
Gokul Eximp
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran



You may like
-
 89-25-8 98%View Details
89-25-8 98%View Details
89-25-8 -
 Edaravone 98%View Details
Edaravone 98%View Details -
 3-Methyl-1-phenyl-2-pyrazolin-5-one, 99% CAS 89-25-8View Details
3-Methyl-1-phenyl-2-pyrazolin-5-one, 99% CAS 89-25-8View Details
89-25-8 -
 3-Methyl 1-phenyl-5-pyrazolone CAS 89-25-8View Details
3-Methyl 1-phenyl-5-pyrazolone CAS 89-25-8View Details
89-25-8 -
 3-Methyl-1-phenyl-5-pyrazolone CAS 89-25-8View Details
3-Methyl-1-phenyl-5-pyrazolone CAS 89-25-8View Details
89-25-8 -
 Antipyrine Related Compound A >99% (GC) CAS 89-25-8View Details
Antipyrine Related Compound A >99% (GC) CAS 89-25-8View Details
89-25-8 -
 1-PHENYL-3-METHYL-5-PYRAZALONE, For IndustrialView Details
1-PHENYL-3-METHYL-5-PYRAZALONE, For IndustrialView Details
89-25-8 -
 1 PHENYL 3 METHYL 5 PYRAZOLONE ( PMP )View Details
1 PHENYL 3 METHYL 5 PYRAZOLONE ( PMP )View Details
89-25-8
