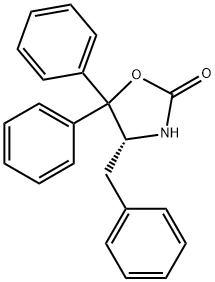
(4R,5S)-(+)-4-METHYL-5-PHENYL-2-OXAZOLIDINONE
Synonym(s):(4R,5S)-4-Methyl-5-phenyl-2-oxazolidinone
- CAS NO.:77943-39-6
- Empirical Formula: C10H11NO2
- Molecular Weight: 177.2
- MDL number: MFCD00010845
- EINECS: 660-040-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:22:11
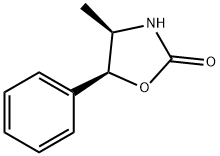
What is (4R,5S)-(+)-4-METHYL-5-PHENYL-2-OXAZOLIDINONE?
Chemical properties
white to light yellow crystal powde
The Uses of (4R,5S)-(+)-4-METHYL-5-PHENYL-2-OXAZOLIDINONE
(4R,5S)-4-Methyl-5-phenyloxazolidinone is used as effective chiral auxiliary for conjugate addition asymmetric synthesis of (-)-aplysillamide B.
The Uses of (4R,5S)-(+)-4-METHYL-5-PHENYL-2-OXAZOLIDINONE
Evan′s chiral auxiliary (4R,5S)-(+)-4-Methyl-5-phenyl-2-oxazolidinone reacts with carboxylic acids to produce corresponding acyl derivatives in the presence of a diisopropylcarbodiimide reagent. It can also employed in the preparation of N-sulfinyloxazolidinone reagent (chiral sulfinyl transfer reagent), which reacts with nucleophiles such as Grignard reagents, enolates, and metalated amides to produce the chiral sulfoxides, sulfinate esters, and sulfonamides.
What are the applications of Application
(4R,5S)-(+)-4-Methyl-5-phenyl-2-oxazolidinone is a chiral auxiliary in condensation reactions with acyl halides and carboxylic acids.
Preparation
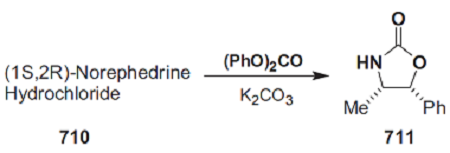
To a solution of (1S,2R)-norephedrine (40 g, 0.26 mol) in toluene (400 mL) was added diethyl carbonate (37 mL, 0.32 mol). The mixture was heated to reflux (under Ar) while 40 mL of solvent was removed through the use of a Dean–Stark apparatus. The mixture was allowed to cool for 20 min, and then sodium methoxide (1 g) was added. Upon reheating, an EtOH/toluene azeotropic mixture was removed at 75–77 °C. After 3 h, the reaction was complete and the temperature of the mixture had increased to 125 °C. The mixture was left to stand at room temperature for 16 h, whereupon (4R,5S)-4-methyl-5-phenyloxazolidin-2-one (40.6 g) crystallized and could be collected. The solvent was removed from the filtrate in vacuo and the residue was redissolved in EtOAc (250 mL). This solution was washed with brine (50 mL) and a precipitate was removed by filtration. The solvent was then removed in vacuo and toluene (50 mL) was added to the residue. Removal of the toluene by distillation yielded oily crystals of the oxazolidinone, which were washed with Et2O to afford 4.5 g (total 45 g, 97%).
Preparation
A mechanically stirred mixture of (1S,2R)-norephedrine 710 (151 g, 1.00 mol) ([α]589 =+ 33.4 (c= 7, water)), as the hydrochloride salt, diphenyl carbonate (236 g, 1.10 mol), and anhydrous potassium carbonate (152 g, 1.10 mol) was heated at 110 °C for 4–6 h. The resultant mixture was then cooled to <60 °C. Excess diphenyl carbonate was hydrolyzed by adding methanol (600 mL) and heating the mixture under reflux for 0.5 h. Sufficient water (400–600 mL) was then added to dissolve the potassium carbonate. Methanol was removed in vacuo. The product and phenol were extracted into dichloromethane (3 × 1 L). The combined extracts were washed with 2 m aqueous sodium hydroxide (3 × 1 L) to remove the phenol, 1 m aqueous hydrochloric acid (1 × 1 L), and brine, dried over anhydrous magnesium sulfate, and concentrated in vacuo to give 195 g (110% mass balance) of a light-yellow solid. Recrystallization from toluene (600 mL) afforded 145–165 g (82–93%) of oxazolidinone 711 as a white crystalline solid.
Definition
ChEBI: (4R,5S)-(+)-4-Methyl-5-phenyl-2-oxazolidinone is a member of benzenes.
Synthesis Reference(s)
Tetrahedron Letters, 40, p. 6059, 1999 DOI: 10.1016/S0040-4039(99)01256-3
Properties of (4R,5S)-(+)-4-METHYL-5-PHENYL-2-OXAZOLIDINONE
| Melting point: | 121-123 °C(lit.) |
| Boiling point: | 309.12°C (rough estimate) |
| Density | 1.1607 (rough estimate) |
| refractive index | 1.5168 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Chloroform (Slightly), Ethyl Acetate (Slightly) |
| form | Liquid |
| pka | 12.35±0.60(Predicted) |
| color | Clear colorless to yellow-brown, may darken in storage |
| optical activity | [α]18/D +168°, c = 2 in chloroform |
| BRN | 1211705 |
| InChI | InChI=1S/C10H11NO2/c1-7-9(13-10(12)11-7)8-5-3-2-4-6-8/h2-7,9H,1H3,(H,11,12)/t7-,9-/m1/s1 |
| CAS DataBase Reference | 77943-39-6(CAS DataBase Reference) |
Safety information for (4R,5S)-(+)-4-METHYL-5-PHENYL-2-OXAZOLIDINONE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for (4R,5S)-(+)-4-METHYL-5-PHENYL-2-OXAZOLIDINONE
| InChIKey | PPIBJOQGAJBQDF-VXNVDRBHSA-N |
| SMILES | O1[C@@H](C2=CC=CC=C2)[C@@H](C)NC1=O |
(4R,5S)-(+)-4-METHYL-5-PHENYL-2-OXAZOLIDINONE manufacturer
EMBIO LIMITED
New Products
DL-beta-(3-Bromophenyl)alanine 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate Amlodipine EP Impurity-C Budesonide Impurity-K/Budesonide 21-Acetate (Epimers) Gabapentine Impurity 72 Bicalutamide Impurity-B Betahistine EP Impurity C Carbamazepine EP Impurity G 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 3-Hydroxypropionitrile 4-Bromo Benzylcyanide valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran

You may like
-
 (4R,5S)-4-Methyl-5-phenyl-2-oxazolidinone 99%View Details
(4R,5S)-4-Methyl-5-phenyl-2-oxazolidinone 99%View Details -
 77943-39-6 99%View Details
77943-39-6 99%View Details
77943-39-6 -
 (4R,5S)-(+)-4-Methyl-5-phenyl-2-oxazolidinone CAS 77943-39-6View Details
(4R,5S)-(+)-4-Methyl-5-phenyl-2-oxazolidinone CAS 77943-39-6View Details
77943-39-6 -
 110-59-8 valeronitrile 99%View Details
110-59-8 valeronitrile 99%View Details
110-59-8 -
 93-17-4 99%View Details
93-17-4 99%View Details
93-17-4 -
 1529-41-5 3-chlorobenzyl cyanide 99%View Details
1529-41-5 3-chlorobenzyl cyanide 99%View Details
1529-41-5 -
 2856-63-5 99%View Details
2856-63-5 99%View Details
2856-63-5 -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5
