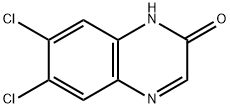
2,3-Dichloroquinoxaline
- CAS NO.:2213-63-0
- Empirical Formula: C8H4Cl2N2
- Molecular Weight: 199.04
- MDL number: MFCD00006720
- EINECS: 218-667-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:21:58
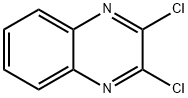
What is 2,3-Dichloroquinoxaline?
Description
2,3-Dichloroquinoxaline (DCQX) can be used as a substrate for nucleophilic aromatic substitution (SNAr) reactions and is a versatile building block for the synthesis of many quinoxaline derivatives. 2,3-Dichloroquinoxaline reacts with 6-aminothiouracil in ethanol/TEA to form 6-amino-2-(3-chloroquinoxalin-2-ylthio)pyrimidin-4(3H)-one. It reacts with cholest-5-en-3-one semicarbazone/thiosemicarbazone to form steriodal cholest-5-en-3-oxazolo and thiazoloquinoxaline.
Chemical properties
solid
The Uses of 2,3-Dichloroquinoxaline
2,3-Dichloroquinoxaline is a dichlorinated quinoxaline derivative with antimicrobial activity. 2,3-Dichloroquinoxaline is used in the preparation of quinoxaline derivatives of cancer chemopreventive a ctivity.
The Uses of 2,3-Dichloroquinoxaline
2,3-Dichloroquinoxaline was used in the synthesis of mono and 2,3-disubstituted quinoxalines. It was used in solid phase synthesis of HPLC chiral stationary phase containing the N,N′-dialkyl-2,3-diaminoquinoxaline group as a linking structure.
Preparation
To a stirred solution of quinoxaline-2,3(1H,4H)-dione (5.00 g, 1.0 equiv.), POCl3 was added (20 ml) and refluxed at 100 °C for 3h. After completion of the reaction, as indicated by TLC, the reaction mass was distilled under vaccum and quenched with ice cold water. Off white semi solid was formed and is filtered through buchner funnel under vaccum to yield the 2,3-dichloroquinoxaline in excellent yield.
Appearance: off white solid; Yield: 92% (5.64g); ESI-MS: (m/z) calcd. for C8H4Cl2N2: 197.98, found: 199.10 [M+H]+.
General Description
Gray solid. Insoluble in water.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
A halogenated amine. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides.
Fire Hazard
2,3-Dichloroquinoxaline is probably combustible.
Purification Methods
Recrystallise it from *C6H6 and dry it in a vacuum [Cheeseman J Chem Soc 1804 1955, Beilstein 23/7 V 144].
Properties of 2,3-Dichloroquinoxaline
| Melting point: | 152-154 °C (lit.) |
| Boiling point: | 325.69°C (rough estimate) |
| Density | 1.4994 (rough estimate) |
| refractive index | 1.6400 (estimate) |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| Water Solubility | Insoluble in water |
| solubility | soluble in Chloroform |
| pka | -4.78±0.48(Predicted) |
| form | Needles |
| color | Off-white to light brown |
| BRN | 126076 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 2213-63-0(CAS DataBase Reference) |
| EPA Substance Registry System | 2,3-Dichloroquinoxaline (2213-63-0) |
Safety information for 2,3-Dichloroquinoxaline
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 2,3-Dichloroquinoxaline
| InChIKey | SPSSDDOTEZKOOV-UHFFFAOYSA-N |
2,3-Dichloroquinoxaline manufacturer
SAKEM LLP
INNOVATIVE LABS
Dr .Silviu Pharmachem Pvt. Ltd.
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile 3,4 Dimethoxy Benzylcyanide valeronitrile 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran




You may like
-
 2213-63-0 2,3-Dichloroquinoxaline 98%View Details
2213-63-0 2,3-Dichloroquinoxaline 98%View Details
2213-63-0 -
 2213-63-0 98%View Details
2213-63-0 98%View Details
2213-63-0 -
 2,3-Dichloroquinoxaline 98%View Details
2,3-Dichloroquinoxaline 98%View Details
2213-63-0 -
 2,3-Dichloroquinoxaline CAS 2213-63-0View Details
2,3-Dichloroquinoxaline CAS 2213-63-0View Details
2213-63-0 -
 2,3-Dichloroquinoxaline CAS 2213-63-0View Details
2,3-Dichloroquinoxaline CAS 2213-63-0View Details
2213-63-0 -
 2,3-Dichloroquinoxaline Compound Or Quinoxaline,2,3-DichloroView Details
2,3-Dichloroquinoxaline Compound Or Quinoxaline,2,3-DichloroView Details
2213-63-0 -
 2,3-Dichloroquinoxaline 2213-63-0 98%View Details
2,3-Dichloroquinoxaline 2213-63-0 98%View Details
2213-63-0 -
 2,3-Dichloroquinoxaline 2213-63-0 99%View Details
2,3-Dichloroquinoxaline 2213-63-0 99%View Details
2213-63-0
