2-Amino-4-phenylbutane
- CAS NO.:22374-89-6
- Empirical Formula: C10H15N
- Molecular Weight: 149.24
- MDL number: MFCD00056185
- EINECS: 244-942-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:14:15
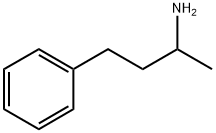
What is 2-Amino-4-phenylbutane?
Description
Amphetamine, or 1-phenyl-2-aminopropane, was first synthesized in 1887 by Romanian chemist L. Edeleanu. It is a central nervous system stimulant and the parent compound of a family of widely used (and abused) psychoactive drugs. It is sold under many trade names—Benzedrine, Dexedrine, and Adderall are the most prominent—and as the racemic mixture or either of its two optical isomers. The?D-isomer is shown.
Chemical properties
clear colorless liquid
The Uses of 2-Amino-4-phenylbutane
2-Amino-4-phenylbutane is used in the synthesis of N-substituted derivatives of (1-methyl-3- phenylpropyl)amine.
Biological Functions
Amphetamine is an indirectly acting adrenomimetic amine that depends for its action on the release of norepinephrine from noradrenergic nerves. Its pharmacological effects are similar to those of ephedrine; however, its CNS stimulant activity is somewhat greater. Both systolic and diastolic blood pressures are increased by oral dosing with amphetamine. The heart rate is frequently slowed reflexively. Cardiac output may remain unchanged in the low- and moderate-dose range. The therapeutic uses of amphetamine are based on its ability to stimulate the CNS. The D-isomer (dextroamphetamine) is three to four times as potent as the L-isomer in producing CNS effects. It has been used in the treatment of obesity because of its anorexic effect, although tolerance to this effect develops rapidly. It prevents or overcomes fatigue and has been used as a CNS stimulant. Amphetamine is no longer recommended for these uses because of its potential for abuse. Amphetamine is useful in certain cases of narcolepsy or minimal brain dysfunction.
Flammability and Explosibility
Not classified
Safety Profile
A poison by intraperitoneal and parenteral route. Moderately toxic by ingestion.When heated to decomposition it emits toxic vapors of NOx.
Synthesis
Amphetamine is prepared by treating phenylacetone with formic acid and ammonia under the Leuckart-Wallach conditions .
Properties of 2-Amino-4-phenylbutane
| Melting point: | -50°C |
| Boiling point: | 228-232 °C (lit.) |
| Density | 0.922 g/mL at 25 °C (lit.) |
| vapor pressure | 10.146Pa at 25℃ |
| refractive index | n |
| Flash point: | 208 °F |
| storage temp. | −20°C |
| solubility | soluble in Chloroform, Ethyl Acetate |
| pka | pKa 10.0 (Uncertain) |
| Specific Gravity | 0.922 |
| color | Clear |
| PH | 11.6 (10g/l, H2O, 20℃) |
| Water Solubility | 8.5 g/L (20 ºC) |
| Sensitive | Air Sensitive |
| BRN | 2413110 |
| CAS DataBase Reference | 22374-89-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzenepropanamine, «alpha»-methyl-(22374-89-6) |
Safety information for 2-Amino-4-phenylbutane
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P270:Do not eat, drink or smoke when using this product. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 2-Amino-4-phenylbutane
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile 3,4 Dimethoxy Benzylcyanide valeronitrile 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran



You may like
-
 22374-89-6 l-Methyl-3-phenylpropylamine 99%View Details
22374-89-6 l-Methyl-3-phenylpropylamine 99%View Details
22374-89-6 -
 1-Methyl-3-phenylpropylamine 98%View Details
1-Methyl-3-phenylpropylamine 98%View Details
22374-89-6 -
 1-Methyl-3-phenylpropylamine CAS 22374-89-6View Details
1-Methyl-3-phenylpropylamine CAS 22374-89-6View Details
22374-89-6 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 5-azidovalericacidView Details
5-azidovalericacidView Details
79583-98-5
