Ensitrelvir
- CAS NO.:2647530-73-0
- Empirical Formula: C22H17ClF3N9O2
- Molecular Weight: 531.88
- Update Date: 2025-12-16 16:15:04
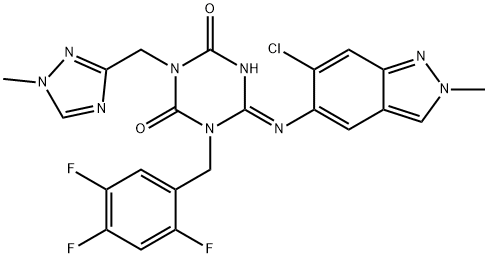
What is Ensitrelvir?
Description
A clinical candidate for treating COVID-19, Ensitrelvir (a nonproprietary name for S-217622), which was discovered through a research collaboration between Shionogi & Co., Ltd. and Hokkaido university, as the first nonpeptidic, noncovalent CLpro inhibitor. Ensitrelvir exhibited significant potent antiviral activity and high bioavailability arising from a favorable drug metabolism and pharmacokinetic (DMPK) profile as a once-daily oral medicine to treat COVID-19.
Mechanism of action
Like Paxlovid, Ensitrelvir also targets the 3CL protease (3CLpro). These two oral drugs are designed to block the activity of the SARS-CoV-2-3CL protease, thereby blocking the replication of the novel coronavirus. It became the first Japanese domestic pill to treat COVID-19 and the third to be regulatorally approved in February 2022 in Japan.[1]
Side Effects
The clinical trial results also showed that after taking Ensiprevir, symptoms such as runny nose, nasal congestion, sore throat, cough, and dyspnea were greatly improved, with minimal side effects. And it has a significant inhibitory effect on the proliferation of the novel coronavirus strain of Omicron.
Synthesis
A practical route to Ensitrelvir: The unprecedented strategy utilizing a meta-cresolyl moiety enhanced the stability of intermediate compounds and enabled scalable manufacturing of 1. The 6-step longest linear sequence (from 26) delivers the target molecule in 35.1% yield. This overall yield was improved by approximately 7-fold compared to the earlier process used in the early research and development stage (4.8%). Our convergent approach to the synthesis of 1 made the manufacturing process concise, streamlined, and short. Direct crystallization was achieved in 9 out of the 12 steps, thus establishing a greener process without silica-gel column chromatographic purification. In addition, the present practical process settled all the issues that made large-scale synthesis difficult, i.e., removal of corrosive acid by evaporation and the generation of noxious odors from thiol derivatives.[2]
Regulations
The U.S. Food and Drug Administration (FDA) granted Fast Track designation to Ensitrelvir for COVID-19. FDA Fast Track designation is designed to facilitate the development and expedite the review of potential new therapies that treat severe conditions and fulfill an unmet medical need. Ensitrelvir was approved in Singapore in November 2023 based on the Special Access Route application. Ensitrelvir remains an investigational drug outside of Japan and Singapore. In addition, the brand name Xocova? has not been approved for use outside of Japan and Singapore and pertains only to the approved drug in Japan and Singapore.
References
[1] YOTSUYANAGI H, OHMAGARI N, DOI Y, et al. Efficacy and Safety of 5-Day Oral Ensitrelvir for Patients With Mild-to-Moderate COVID-19: The SCORPIO-SR Randomized Clinical Trial[C]. 2023: 0. DOI:10.1101/2023.07.11.23292264.
[2] TAKAHIRO KAWAJIRI*, TAKAYUKI TSURITANI Development of a Manufacturing Process toward the Convergent Synthesis of the COVID-19 Antiviral Ensitrelvir[J]. ACS Central Science, 2023, 9 4: 836-843. DOI:10.1021/acscentsci.2c01203.
Properties of Ensitrelvir
| Boiling point: | 696.2±65.0 °C(Predicted) |
| Density | 1.65±0.1 g/cm3(Predicted) |
| solubility | DMSO:160.0(Max Conc. mg/mL);300.82(Max Conc. mM) |
| form | A crystalline solid |
| pka | 5.19±0.20(Predicted) |
| color | White to light yellow |
Safety information for Ensitrelvir
Computed Descriptors for Ensitrelvir
| InChIKey | QMPBBNUOBOFBFS-UHFFFAOYSA-N |
| SMILES | N1(CC2=CC(F)=C(F)C=C2F)C(=NC2C(Cl)=CC3C(C=2)=CN(C)N=3)NC(=O)N(CC2N=CN(C)N=2)C1=O |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidYou may like
-
 S-217622 CAS 2647530-73-0View Details
S-217622 CAS 2647530-73-0View Details
2647530-73-0 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
