1-METHYL-D-TRYPTOPHAN
- CAS NO.:110117-83-4
- Empirical Formula: C12H14N2O2
- Molecular Weight: 218.25
- MDL number: MFCD00274271
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-02-02 18:10:39
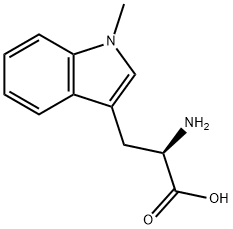
What is 1-METHYL-D-TRYPTOPHAN?
Description
Indoleamine 2,3-dioxygenase (IDO) metabolizes tryptophan to kynurenine, leading to the production of NAD+ via the kynurenine pathway. The overexpression of IDO in tumors and tumor-draining lymph nodes induces immune tolerance and enhances tumor growth in vivo. 1-methyl-D-Tryptophan is an inhibitor of IDO (IC50 = 7 μM) that is effective in vivo. In mice, 1-methyl-D-tryptophan prevents T-cell anergy triggered by dendritic cells overexpressing IDO. It augments the antitumor and antiviral immunoresponse of CD8+ T-cells and reduces tumor volume in mice with xenografts overexpressing IDO. 1-methyl-D-Tryptophan, in combination with chemotherapy, causes regression of tumors and prolongs survival. Surprisingly, 1-methyl-D-tryptophan induces the expression of IDO in human ovarian carcinoma SKOV3 cells in culture.
The Uses of 1-METHYL-D-TRYPTOPHAN
Indoleamine 2,3-dioxygenase (IDO) metabolizes tryptophan to kynurenine, leading to the production of NAD+ via the kynurenine pathway. The overexpression of IDO in tumors and tumor-draining lymph nodes induces immune tolerance and enhances tumor growth in vivo. 1-methyl-D-Tryptophan is an inhibitor of IDO (IC50 = 7 μM) that is effective in vivo. In mice, 1-methyl-D-tryptophan prevents T-cell anergy triggered by dendritic cells overexpressing IDO. It augments the antitumor and antiviral immunoresponse of CD8+ T-cells and reduces tumor volume in mice with xenografts overexpressing IDO. 1-methyl-D-Tryptophan, in combination with chemotherapy, causes regression of tumors and prolongs survival. Surprisingly, 1-methyl-D-tryptophan induces the expression of IDO in human ovarian carcinoma SKOV3 cells in culture.
The Uses of 1-METHYL-D-TRYPTOPHAN
1-Methyl-D-tryptophan is used in biological studies to determine the effect of IDO2 enzyme (indoleamine-(2,3)-dioxygenase) activity and IDO2-mediated arrest of human T cell proliferation. It reverses IDO-mediated immune suppression
What are the applications of Application
1-Methyl-D-tryptophan is an inhibitor of IDO and INDOL1 (IDO2)
Storage
Store at -20°C
References
1) Hou?et al. (2007),?Inhibition of indoleamine 2,3-dioxygenase in dendritic cells by stereoisomers of 1-methyl-tryptophan correlates with antitumor responses; Cancer Res.,?67?792 2) Soliman?et al. (2010),?Indoleamine 2,3-dioxygenase: is it an immune suppressor?; Cancer J.,?16?354 3) Friberg?et al. (2002),?Indoleamine 2,3-dioxygenase contributes to tumor cell evasion of T-cell mediated rejection; Int. J. Cancer,?101?151 4) Metz?et al. (2012),?IDO inhibits a tryptophan sufficiency signal that stimulates mTOR; Oncoimmunol.,?1?1460
Properties of 1-METHYL-D-TRYPTOPHAN
| Melting point: | 242-245 °C(lit.) |
| alpha | 12.4o (C = 2 IN ACETIC ACID) |
| Boiling point: | 429.3±35.0 °C(Predicted) |
| Density | 1.28 |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | Soluble in DMSO (greater than 25 mg/ml). |
| pka | 2.26±0.10(Predicted) |
| form | White to light brown crystalline powder. |
| color | White |
| optical activity | [α]22/D +12.4°, c = 2 in acetic acid |
| Water Solubility | Soluble to 5 mM in water with gentle warming |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20° for up to 1 month. |
Safety information for 1-METHYL-D-TRYPTOPHAN
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 1-METHYL-D-TRYPTOPHAN
| InChIKey | ZADWXFSZEAPBJS-SNVBAGLBSA-N |
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile 3,4 Dimethoxy Benzylcyanide valeronitrile 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran



You may like
-
 1-Methyl-D-tryptophan CAS 110117-83-4View Details
1-Methyl-D-tryptophan CAS 110117-83-4View Details
110117-83-4 -
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5 -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 5-azidovalericacidView Details
5-azidovalericacidView Details
79583-98-5
