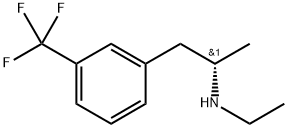
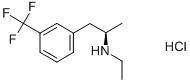
(+)-Fenfluramine hydrochloride
Synonym(s):(+)-N-Ethyl-α-methyl-m-[trifluoromethyl]phenethylamine hydrochloride;Dexfenfluramine hydrochloride
- CAS NO.:3239-45-0
- Empirical Formula: C12H16F3N.ClH
- Molecular Weight: 267.72
- MDL number: MFCD00069276
- EINECS: 221-806-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-06-30 15:45:59
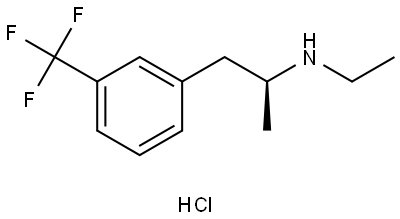
What is (+)-Fenfluramine hydrochloride?
Description
Redux, having been already launched in Europe, was marketed in Canada and the US for obesity. The (+)-isomer of fenfluramine is obtained by resolution using d-camphoric acid and ultimate conversion to the HCl salt. The levo-isomer has significant effects on dopaminerigic neurotransmission, while the dextro-isomer has a greater anorectic effect because it is more selective on serotonin as a 5-HT agonist with no dopaminergic or noradenergic activity. Redux inhibits the presynaptic release of serotonin, is a reuptake inhibitor, a 5-HT1B, receptor agonist, and a postsynaptic 5-HT2C receptor agonist . A dietary reduction in fat intake was thought to proceed via a CCK A-type receptor agonist activity. Due to the side effects of primary pulmonary hypertension, brain serotonin neurotoxicity and valvular heart disease, Redux was withdrawn world wide.
Chemical properties
White Crystalline Powder
Originator
Servier (France)
The Uses of (+)-Fenfluramine hydrochloride
Controlled substance. Anorexic
Definition
ChEBI: The hydrochloride salt of (S)-fenfluramine. It stimulates the release of serotonin and selectively inhibits its reuptake, but unlike the racemate it does not possess catecholamine agonist activity. It was formerly given by mouth in the tre tment of obesity, but, like the racemate, was withdrawn wolrdwide following reports of valvular heart defects.
brand name
Redux (Interneuron).
Biological Activity
Indirectly agonizes serotonin receptors via inhibition of 5-HT re-uptake and stimulation of 5-HT release. Anorectic agent; decreases growth hormone, insulin, leptin, fat mass, lean mass and increases ghrelin in diet-switched diet-induced obese mice.
Biochem/physiol Actions
(+)-Fenfluramine is a serotonin uptake inhibitor; anoxexic. (+)-Fenfluramine is neurotoxic on prolonged administration or at high dosage. (+)-Fenfluramine releases serotonin from axon terminals by a nonexocytotic mechanism.
Storage
Desiccate at RT
Properties of (+)-Fenfluramine hydrochloride
| Melting point: | 159-163°C |
| storage temp. | Desiccate at RT |
| solubility | H2O: soluble10mg/mL |
| form | powder |
| color | white to off-white |
Safety information for (+)-Fenfluramine hydrochloride
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P403+P233:Store in a well-ventilated place. Keep container tightly closed. |
Computed Descriptors for (+)-Fenfluramine hydrochloride
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran



You may like
-
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 1529-41-5 3-chlorobenzyl cyanide 99%View Details
1529-41-5 3-chlorobenzyl cyanide 99%View Details
1529-41-5 -
 2856-63-5 99%View Details
2856-63-5 99%View Details
2856-63-5 -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5 -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8
