- Price
Supplier Type
- Manufacturer
- Reagent
- Trader
- contract manufacturer
- custom synthesis
Supplier Region
- Bangalore(3)
- Gujarat(37)
- Pune(8)
- Maharashtra(34)
- Tamil Nadu(6)
- Delhi(11)
- Mumbai(66)
- Andhra Pradesh(2)
- Karnataka(9)
- Hyderabad(30)
- Ahmedabad(26)
- Beijing(3)
- Vadodara(20)
- Telangana(3)
- New Delhi(4)
- Himachal Pradesh(1)
- Rajasthan(2)
- Madhya Pradesh(1)
- Uttar Pradesh(4)
- Punjab(2)
- Haryana(1)
- Ujjain(1)
- Kolkata(1)
- Mallapur(1)
- Nagpur(1)
- GOA(1)
Purity
- Upto 90%
- Salicylic Acid
- Rasagiline Mesylate Impurity C
- more than 95%
- more than 90%
- MINIMUM 99%
- Min. 98%
- Min. 98 %
- Min. 80%
- Min. 47.9 % W/W
- MIN 99%
- Min 98. 00%
- Mesalazine EP Impurity H
- Mathadone (M225865) impurity. Used in synthesis of methadone, antispasmodics and other pharmaceutica
- high
- Greater than 99%
- Empagliflozin intermediate, Dapagliflozin Impurity
- Dipropargyl r- amino indane
- Dapagliflozin Intermediate, Dapagliflozin Impurity
- Boron 20%
- API Impurity / In-house working standard
- Acetylsalicylic Acid EP Impurity C
- Above 80%
- 99.99%
- 99.98%
- 99.95%
- 99.94%
- 99.91%
- 99.9% min
- >99.9%
- 99.9%
- 99.86%
- 99.85%
- 99.82%
- 99.80%
- 99.76%
- 99.73%
- ≥ 99.5%
- 99.42%
- 99.29%
- 99.18%
- 99.00%
- 99%+
- 99%
- >99%
- 98+
- 98.86%
- 98.80%
- 98.60%
- 98.12%
- 98.00%
- 98.0%
- 98%+
- 98% +
- 98%
- >98%
- 98 %
- 97-99%
- 97.57%
- 97.00%
- 97%
- >97%
- 97 %
- 96.80%
- 96.56%
- 96.39%
- 96.14%
- 96%
- 95-98%
- 95.48%
- 95.00%
- >95%
- 95%
- 94.00%
- >92%
- >90%
- 90%
- 90 % Above
- 80%
- 7%,9% & 11%
- 65%
- 52
- 50%
- 50 % and 80%
- 50 %
- 5% SG
- 5 %
- 40% & 0.5M
- 39 -41%
- 30%
- 25%
- 20%~98% Aescin
- 20 %
- 1.0M/2.0M/ 7.0M
- 1.0M
- (R)-N,N-di(prop-2-yn-1-yl)-2,3- dihydro-1
Package
- 0MG TO GRAM
- 100mg
- 5g
- 10g
- 25gm
- 50gm
- 100
- 200
- 25
- 50
- 25mg
- 50mg
- 250mg
- 500mg
- 1gm
- 11Between 1 gm to 1 MT (As per availability)
- 1g
- 25g
- 50g
- 100ml
- 100g
- 100gm
- 200gm
- 250gm
- 500gm
- 500g
- 500ml
- 1kg
- 1L
- 5kg
- 10kg
- 25kg
- 50kg
- 100kg
- 500kg
- 1MT
- 1and gm
- 50 gms
- 100 gms
- 250kgs
- 500gms
- 250250 mg.
-
(S)-2-(2-(5-chloro-N-(4-(5-((5-chlorothiophene-2-carboxamido)methyl)-2-oxooxazolidin-3-yl)phenyl)thiophene-2-carboxamido)ethoxy)acetic acid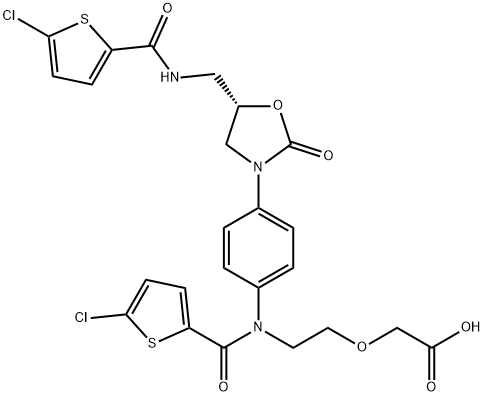
1151893-81-0
-
1H-ISOINDOLE-1,3(2H)-DIONE, 2-[[(5S)-2-OXO-3-[4-(3-OXO-4-MORPHOLINYL)PHENYL]-5-OXAZOLIDINYL]METHYL]-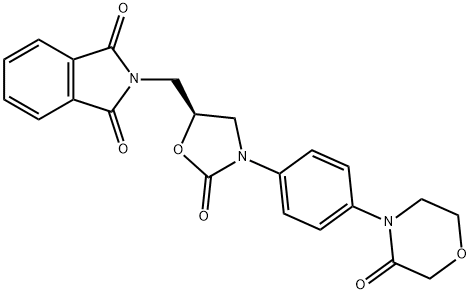
446292-08-6
-
Rivaroxaban Impurity 25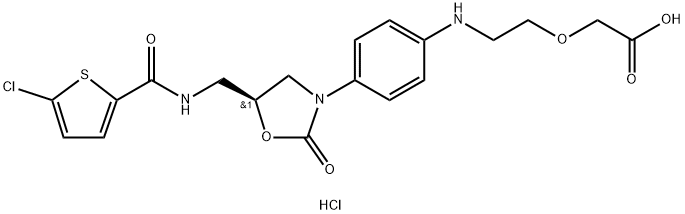
931117-61-2
-
1,3-bis(4-(3-oxomorpholino)phenyl)urea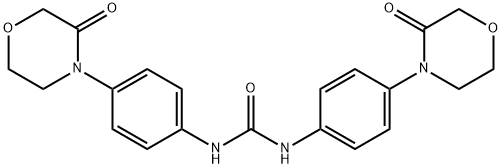
1855920-51-2
-
3-MORPHOLINONE, 4-[4-[(5S)-5-(AMINOMETHYL)-2-OXO-3-OXAZOLIDINYL]PHENYL]-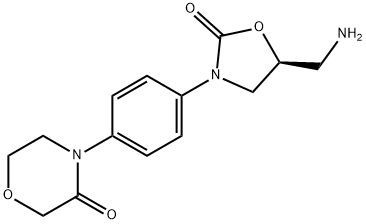
446292-10-0
-
Rivaroxaban Impurity 27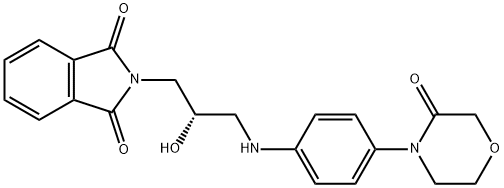
1369969-44-7
-
2-ThiophenecarboxaMide, N-[[(5S)-2-oxo-3-[4-(3-oxo-4-Morpholinyl)phenyl]-5-oxazolidinyl]Methyl]-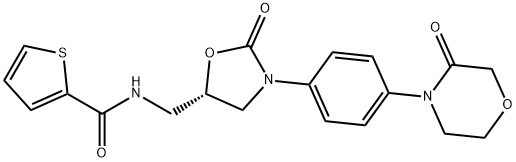
1415566-28-7
-
(S)-4,5-dichloro-N-((2-oxo-3-(4-(3-oxoMorpholino)phenyl)oxazolidin-5-yl)Methyl)thiophene-2-carboxaMide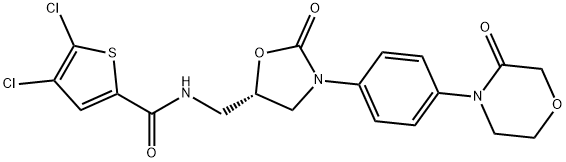
1770812-37-7
-
Urea, N,N'-bis[[(5S)-2-oxo-3-[4-(3-oxo-4-Morpholinyl)phenyl]-5-oxazolidinyl]Methyl]-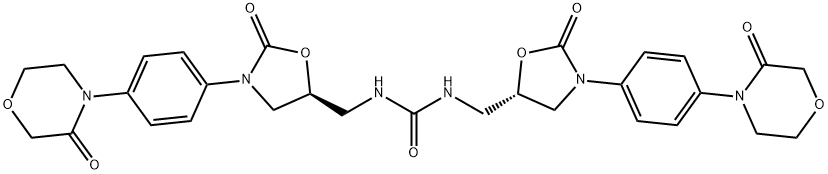
1365267-35-1
-
Benzoic acid, 2-[[[[(5S)-2-oxo-3-[4-(3-oxo-4-Morpholinyl)phenyl]-5-oxazolidinyl]Methyl]aMino]carbonyl]-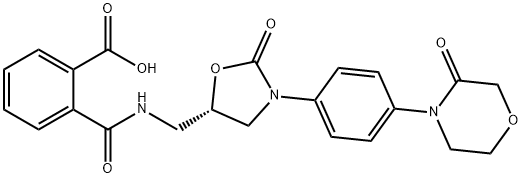
1365267-37-3
Rivaroxaban - Impurity 1151893-81-0
RIVAROXABAN IMPURITY 30 1369969-44-7
99% Azithromycin Impurity J 117693-41-1 API Powder, Lab Grade 117693-41-1

Calcium-sulfate dihydrate 10101-41-4

Deferasirox 201530-41-8

L-Alanine 56-41-7

Benzalacetophenone 94-41-7

Pigment red 48:1 7585-41-3

Methacrylic acid 79-41-4





