- Price
Supplier Type
- Manufacturer
- contract manufacturer
Supplier Region
- Gujarat(1)
- Karnataka(1)
Purity
- United States Pharmacopeia (USP) Reference Standard
- Pharmaceutical Secondary Standard
- European Pharmacopoeia (EP) Reference Standard
- Certified Reference Material
- analytical standard
- 90 % Above
Package
- 5mg
- 15mg
- 25mg
- 30mg
- 100mg
- 250mg
- 500mg
- 1gm
- 1g
- 18%
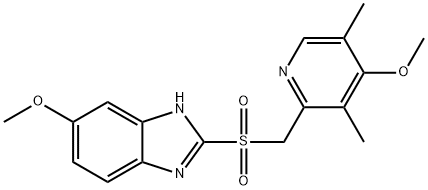
OMEPRAZOLE RELATED COMPOUND A (15 MG) (OMEPRAZOLE SULFONE) (AS)
- CAS:88546-55-8
- MF:C17H19N3O4S
- MW:361.42
- Melting point::
- 80-82°C
- Boiling point::
- 599.3±60.0 °C(Predicted)
- Density :
- 1.331±0.06 g/cm3(Predicted)
- storage temp. :
- Inert atmosphere,2-8°C
- solubility :
- ≥40.6 mg/mL in DMSO,≥19.9 mg/mL in EtOH,insoluble in H2O
- form :
- neat
- pka:
- 7.97±0.10(Predicted)
- form :
- Solid
- color :
- White to off-white
- BRN :
- 8347309
- Symbol(GHS)

GHS07
- Signal word
- Warning
- Hazard statements
- H315-H319-H335
- Precautionary statements
- P261-P264-P271-P280-P302+P352-P305+P351+P338
OMEPRAZOLE EP IMPURITY D 88546-55-8

Omeprazole sulfone 88546-55-8
Omeprazole impurity D 88546-55-8
 Inquiry
Inquiry
Omeprazole sulfone 95% 88546-55-8
 1week
1week