Supplier Type
- Manufacturer
- custom synthesis
- Trader
- Reagent
- contract manufacturer
Supplier Region
- Gujarat(40)
- New Delhi(6)
- Mumbai(76)
- Maharashtra(29)
- Rajasthan(2)
- Andhra Pradesh(1)
- Karnataka(10)
- Hyderabad(23)
- Bangalore(2)
- Telangana(2)
- Delhi(22)
- Ahmedabad(21)
- Pune(8)
- Vadodara(17)
- Haryana(5)
- Uttar Pradesh(11)
- Madhya Pradesh(4)
- Punjab(4)
- Kolkata(6)
- Tamil Nadu(2)
- Nagpur(4)
- Himachal Pradesh(1)
Purity
- yes
- Salicylic Acid
- Rasagiline Mesylate Impurity C
- Purity
- NLT 99%
- N/A
- More than 98%
- more than 95%
- more than 90%
- MINIMUM 99%
- Min.96%
- Min. 35.0%
- Min 98 %
- Mesalazine EP Impurity H
- Mathadone (M225865) impurity. Used in synthesis of methadone, antispasmodics and other pharmaceutica
- Magnesium content 8.49% to 8.84%
- Magnesium content 4.0-5.0%
- lemon-lime grade (purity, 70%), a clear water-white ...
- Greater than 99%
- Empagliflozin intermediate, Dapagliflozin Impurity
- Dipropargyl r- amino indane
- Dapagliflozin Intermediate, Dapagliflozin Impurity
- CLASS A
- Chemically pure
- Bismuth Content 56.0 %
- API Impurity / In-house working standard
- Acetylsalicylic Acid EP Impurity C
- A Grade
- A
- 99.99%
- 99.98%
- 99.95%
- 99.91%
- 99.90%
- 99.9%
- 99.9
- 99.88%
- 99.86%
- 99.85%
- 99.84%
- 99.81%
- 99.80%
- 99.8%
- 99.73%
- 99.70%
- 99.69%
- 99.61%
- 99.6
- 99.55%
- 99.50%
- 99.5% to 100.5%
- 99.5%
- 99.47%
- 99.42%
- 99.40%
- 99.39%
- 99.29%
- 99.28%
- 99.27%
- 99.21%
- 99.19%
- 99.10%
- 99.00%
- 99.0%
- 99.0 &100.5
- 99.0 % - 101.0 %
- 99% Pure
- 99% Minimum
- 99% min
- 99%
- > 99%
- >99%
- 99 Percent
- 99 %
- 99
- 98-99%
- 98=%
- 98+
- 98.94%
- 98.88%
- 98.65%
- 98.60%
- 98.54%
- 98.53%
- 98.50%
- 98.5 to 101.5%
- 98.5
- 98.34%
- 98.18%
- 98.12%
- 98.08%
- 98.00%
- 98.00 % - 102.00 %
- 98.0% to 101.0%
- 98.0% - 102.0%
- 98.0%
- 98.0 % -102.0 %
- 98.0 % - 100.5 %
- 98.0 - 100.5%
- 98% Minimum
- 98% Min.
- 98% Min
- >98%
- 98%
- 98 % min
- 97-99%
- 97.84%
- 97.61%
- 97.57%
- > 97.5 %
- 97.00%
- 97.0%min,97%min
- 97% min
- >97%
- 97%
- 97 %
- 96.80%
- > 96.5 %
- 96.5 - 103.5 %
- 96.40%
- 96.39%
- 96.14%
- 96.00 % Min
- 96%
- 96 PURITY
- 95-98%
- 95.48%
- 95.16%
- 95.00%
- 95.00 % Min
- 95% min
- 95%
- >95%
- 95 %
- 94.34%
- 94.00%
- 93.0 % Min
- 93 %- 101%
- 92-95%
- >92%
- 91.87%
- 90-95%
- 90%-95%
- ≥90%
- 90%
- >90%
- 90 % Min.
- 90 % Above
- 89%
- 86.0 % - 89.0 %
- 85 percent Min
- 84.49%
- 80
- 75 %
- 68.0 % Min
- 65%
- 52
- 40
- 34%
- 17%
- 14.5 - 16.0%
- 100.00%
- 100% Pure
- 100%
- 0.04%
- (R)-N,N-di(prop-2-yn-1-yl)-2,3- dihydro-1
Package
- 0MG TO GRAM
- 25mg
- 50mg
- 100mg
- 250mg
- 500mg
- 1gm
- 1g
- 1ml
- 5g
- 10g
- 25g
- 50g
- 100g
- 500g
- 1kg
- 5kg
- 10kg
- 25kg
- 50kg
- 100kg
- 500kg
- 1MT
- 1and gm
- 250250 mg.
- 11Between 1 gm to 1 MT (As per availability)
-
2-Acetamidophenol
614-80-2
-
LANSOPRAZOLE SULFONE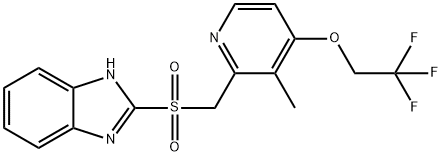
131926-99-3
-
((3AS,5R,6S,6aS)-6-hydroxy-2,2-dimethyltetrahydrofuro[2,3-d][1,3]dioxol-5-yl)(morpholino)methanone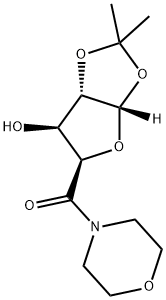
1103738-19-7
-
Montelukast Acyl-b-D-glucuronide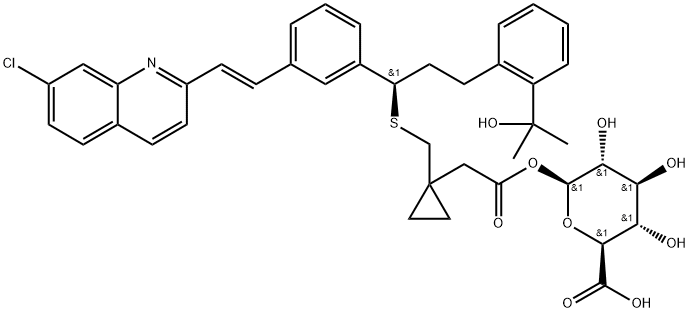
188717-17-1
-
ALPHAZURINE A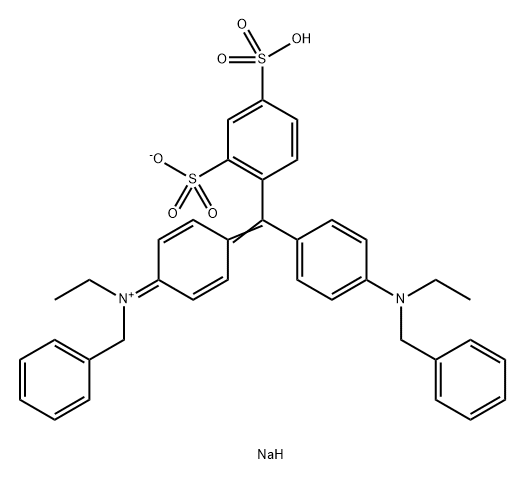
3486-30-4
-
Montelukast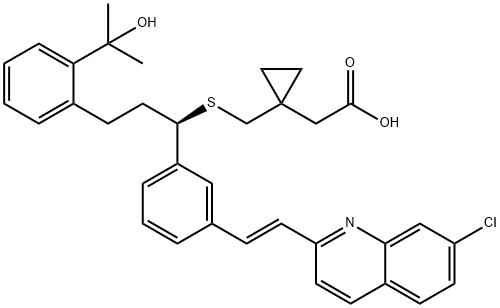
158966-92-8
-
6-HYDROXY-2,4,5-TRIAMINOPYRIMIDINE SULFATE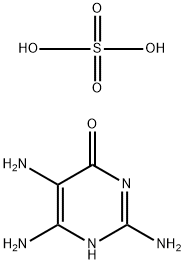
39267-74-8
-
4,6-Dichloro-5-(2-methoxyphenoxy)-2,2'-bipyrimidine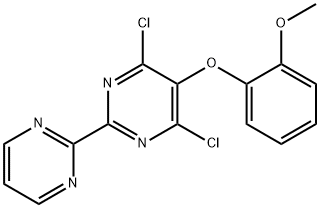
150728-13-5
-
Cyclopropylacetylene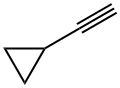
6746-94-7
-
1,2,4-Triazole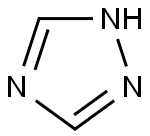
288-88-0
PARACETAMOL EP IMPURITY A/ACETAMINOPHEN USP RELATED COMPOUND C 614-80-2

(5S)-D-xylo-Pentodialdo-5,2-furanose, 4,5-O-(1-Methylethylidene)-1-C-4-Morpholinyl 1103738-19-7

Montelukast acyl-b-D-glucuronide 188717-17-1

Alphazurine A 3486-30-4

Montelukast 158966-92-8

6-Hydroxy-2,4,5-triaminopyrimidine sulfate 39267-74-8

Bisphenol A 80-05-7

4,5,6,7-Tetrahydrothieno[3,2,c]pyridine hydrochloride 28783-41-7

Montelukast sodium 151767-02-1

6-mercapto-6,7-dihydro-5H-pyrazolo-1,2-a-1,2,4-triazol-4-ylium chloride 153851-71-9


