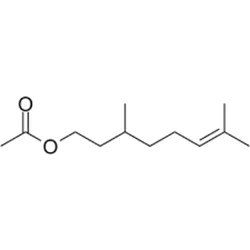
Supplier Type
- Manufacturer
- Reagent
- Trader
- contract manufacturer
Supplier Region
- Bangalore(1)
- Gujarat(7)
- Maharashtra(5)
- Pune(4)
- Hyderabad(4)
- Mumbai(21)
- Karnataka(2)
- Delhi(9)
- Uttar Pradesh(2)
- Vadodara(3)
- Ahmedabad(5)
- New Delhi(1)
- Kolkata(1)
- Madhya Pradesh(1)
- Nagpur(1)
- Haryana(1)
Purity
- Salicylic Acid
- More than 95%
- more than 90%
- Mesalazine EP Impurity H
- Magnesium content 8.49% to 8.84%
- Lab Grade
- Greater than 99%
- API Impurity / in-house working standard
- Acetylsalicylic Acid EP Impurity C
- 99.98%
- 99.95%
- 99.91%
- 99.85%
- 99.8%
- 99.73%
- 99.50%
- 99.42%
- 99.29%
- 99% min
- 99%
- >99%
- 98.60%
- 98.5 to 101.5%
- 98.12%
- 98.0% to 101.0%
- 98.0 % -102.0 %
- 98%
- 98 %
- 97.57%
- 97%
- 97 %
- 96.80%
- 96.39%
- 96.14%
- 96%
- 96 %
- 95.48%
- 95.00%
- 95% min
- 95%
- >95%
- 94.00%
- 94%
- 93 %- 101%
- >92%
- >90%
- 90 % Above
- 67.0% Min. (M.W. 383)
- 50%
- 36%
- 10%
Package
- 25mg
- 50mg
- 100mg
- 250mg
- 500mg
- 1gm
- 1kg
- 5kg
- 10kg
- 25kg
- 1and gm
- 1MT
- 250250 mg.
-
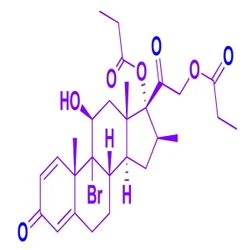
Atorvastatin Methyl Ester
345891-62-5
-
N-(2,6-Dimethylphenyl)-2-(ethylmethylamino)acetamide Hydrochloride_x000b_(Lidocaine Impurity E)
50295-20-0
-
Simvastatin EP Impurity K
-
Ramiprilat Diketopiperazine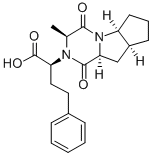
108736-10-3
-
1,1′-Trimethylenedi-theobromine
74857-22-0
-
Chlorhexidine Digluconate IMpurity K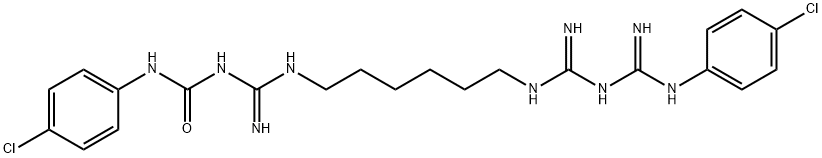
1381962-77-1
-
18-Methyl Nandrolone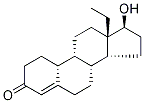
793-55-5
-
4-Aminophenol
123-30-8
-
Beclometasone EP Imp D, CAS:52092-14-5
-
Acyclovir N-Methylene Dimer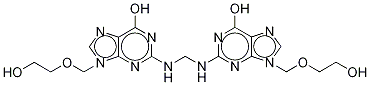
1797131-64-6
Atorvastatin ep impurity K 345891-62-5
LIDOCAINE EP IMPURITY K 50295-20-0
Levonorgestrel EP Impurity K 793-55-5
>92% Aciclovir EP Impurity K, 50mg, Industrial Grade 1797131-64-6