Supplier Type
- Manufacturer
- Trader
- contract manufacturer
- Reagent
- custom synthesis
Supplier Region
- Gujarat(41)
- Maharashtra(31)
- Delhi(13)
- Karnataka(7)
- Ahmedabad(22)
- Vadodara(10)
- New Delhi(8)
- Pune(5)
- Mumbai(48)
- Hyderabad(26)
- Andhra Pradesh(3)
- Kolkata(4)
- Uttar Pradesh(5)
- Bangalore(3)
- Haryana(2)
- Punjab(3)
- Telangana(2)
- Beijing(2)
- Tamil Nadu(3)
- Rajasthan(1)
- Nagpur(1)
- Kerela(1)
Purity
- Salicylic Acid
- Rasagiline Mesylate Impurity C
- NLT 95%
- NLT 90%
- more than 95%
- more than 90%
- Min. 14 %
- Mesalazine EP Impurity H
- Mathadone (M225865) impurity. Used in synthesis of methadone, antispasmodics and other pharmaceutica
- Magnesium content Min. 14%
- Heat resistant injection stage
- Greater than 99%
- Empagliflozin intermediate, Dapagliflozin Impurity
- Dipropargyl r- amino indane
- Dapagliflozin Intermediate, Dapagliflozin Impurity
- API Impurity / In-house working standard
- Acetylsalicylic Acid EP Impurity C
- 99.99%
- 99.98%
- 99.95%
- 99.91%
- 99.90%
- 99.86%
- 99.85%
- 99.80%
- 99.73%
- 99.55%
- 99.50%
- 99.5% min
- 99.5%
- 99.42%
- 99.29%
- 99.26%
- 99.17%
- 99.00%
- 99% Min
- 99% HPLC
- 99%
- > 99%
- >99%
- 99 %
- 98-99%
- 98+
- 98.70%
- 98.60%
- 98.58%
- 98.57%
- 98.42%
- 98.33%
- 98.31%
- 98.12%
- 98.00%
- 98% Min
- 98%
- >98%
- 98 %
- 97-99%
- 97.57%
- 97.00%
- 97%
- >97%
- 97 %
- 96.80%
- 96.40%
- 96.39%
- 96.14%
- 96.00%
- 96%
- 95.48%
- 95.00%
- >95%
- 95%
- 94.00%
- >92%
- 91.21%
- 90%
- >90%
- 90 % Above
- 100 +/- 5%
- 0.5M
- (R)-N,N-di(prop-2-yn-1-yl)-2,3- dihydro-1
Package
- 100mg
- 50
- 25mg
- 250mg
- 500mg
- 25
- 200
- 100
- 0MG TO GRAM
- 2.5501e+00925 MG 50 MG 100 MG 250 MG EXTRA
- 50mg
- 1g
- 1gm
- 5g
- 10g
- 25G
- 50g
- 100ml
- 100g
- 100gm
- 500ml
- 500g
- 1L
- 1kg
- 1000g
- 5kg
- 10kg
- 25kg
- 50kg
- 100kg
- 500kg
- 1MT
- 1and gm
- 250kgs
- 250250 mg.
-
7-Azabicyclo[4.1.0]hept-3-ene-3-carboxylic acid, 7-(1,1-dimethylethyl)-5-(1-ethylpropoxy)-, ethyl ester, (1R,5R,6R)-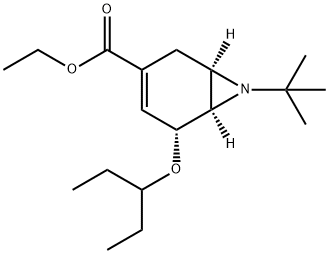
2417645-75-9
-
[1,1'-Biphenyl]-2-carboxamide, 4'-(azidomethyl)-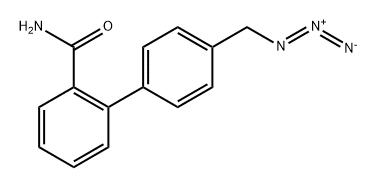
2763750-28-1
-
4-HYDROXY-N,N,2-TRIMETHYL-1H-BENZO[D]IMIDAZOLE-6-CARBOXAMIDE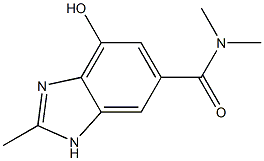
2168520-25-8
-
Carfilzomib Impurity 14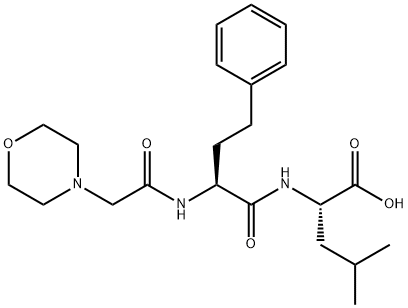
1396006-47-5
-
3-Deschloro-4-Desfluoro-4-Chloro-3-Fluorogefitinib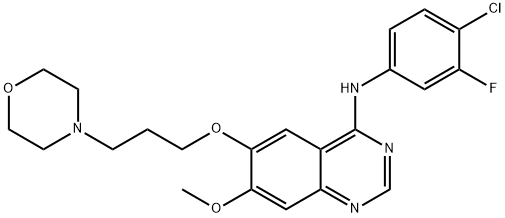
1603814-04-5
-
2,6-Ntp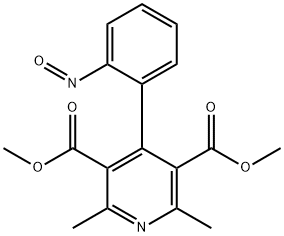
50428-14-3
-
Quetiapine Hydroxy Impurity Dihydrochloride Salt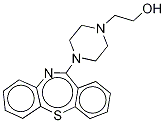
329218-14-6
-
Nebivolol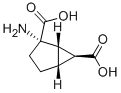
118457-14-0
-
Loxoprofen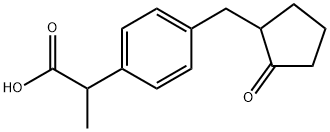
68767-14-6
-
Maltol isobutyrate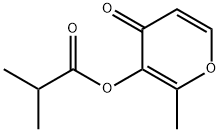
65416-14-0
OSELTAMIVIR IMPURITY 14 2417645-75-9
Carfilzomib Impurity 14 (SS) 1396006-47-5

Nebivolol 118457-14-0

Loxoprofen 68767-14-6

Maltyl isobutyrate 65416-14-0

3-Chloroperoxybenzoic acid 937-14-4

Sodium chloride 7647-14-5

Reactive Orange 12 (C.I 13248) 70161-14-7

Potassium hydrogen carbonate 298-14-6

Reactive Orange 12 (C.I 13248) 70161-14-7



