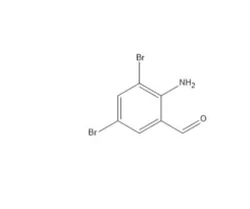
Supplier Type
- Manufacturer
- Reagent
Supplier Region
- Maharashtra(1)
- Hyderabad(7)
- Gujarat(3)
- Bangalore(1)
- Pune(2)
- Mumbai(3)
- Karnataka(1)
- Ahmedabad(1)
- Nagpur(1)
- Punjab(1)
Purity
- Salicylic Acid
- Rasagiline Mesylate Impurity C
- NLT 95%
- NLT 90%
- more than 95%
- more than 90%
- Mesalazine EP Impurity H
- Mathadone (M225865) impurity. Used in synthesis of methadone, antispasmodics and other pharmaceutica
- Greater than 99%
- Empagliflozin intermediate, Dapagliflozin Impurity
- Dipropargyl r- amino indane
- Dapagliflozin Intermediate, Dapagliflozin Impurity
- API Impurity / In-house working standard
- Acetylsalicylic Acid EP Impurity C
- 99.99%
- 99.98%
- 99.95%
- 99.91%
- 99.86%
- 99.85%
- 99.80%
- 99.73%
- 99.42%
- 99.29%
- 99%
- 98.60%
- 98.12%
- 98%
- 97-99%
- 97.57%
- 97%
- 97 %
- 96.80%
- 96.39%
- 96.14%
- 96%
- 95.48%
- 95.00%
- 95%
- >95%
- 94.00%
- >92%
- 90%
- >90%
- 90 % Above
- 25 MG 50 MG 100 MG 250 MG EXTRA
- (R)-N,N-di(prop-2-yn-1-yl)-2,3- dihydro-1
Package
- 25mg
- 50mg
- 100mg
- 250mg
- 500mg
- 1g
- 1gm
- 5g
- 25g
- 1kg
- 5kg
- 10kg
- 1MT
- 250250 mg.
- 2.5501e+00925 MG 50 MG 100 MG 250 MG EXTRA
- 25kg
- 1and gm
- 95NLT %
-
Cabotegravir Isomer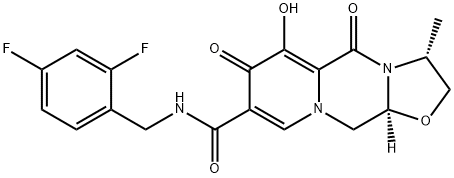
1309560-64-2
-
2,6-Difluoro Cabotegravir Impurity
-
Cabotegravir Diastereomer-2 Impurity
-
Oxazolo[3,2-a]pyrido[1,2-d]pyrazine-8-carboxamide, 2,3,5,7,11,11a-hexahydro-6-hydroxy-3-methyl-5,7-dioxo-N-(phenylmethyl)-, (3S,11aR)-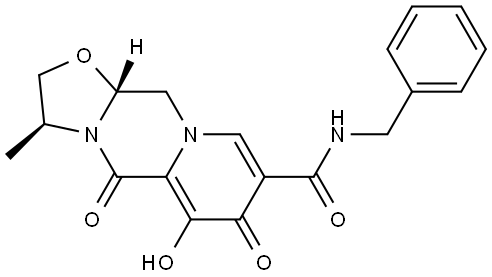
2883234-41-9
-
(S)-N-(2,4-difluorobenzyl)-9-hydroxy-2-(1-hydroxypropan-2-yl)-1,8-dioxo-1,8-dihydro-2H-pyrido[1,2-a]pyrazine-7-carboxamide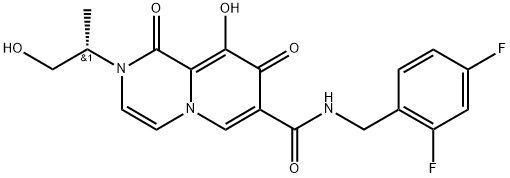
2883234-39-5
-
Brivaracetam (alfaR, 4S)-Isomer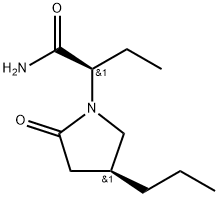
357336-99-3
-
Minocycline hydrochloride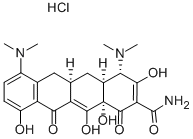
13614-98-7
-
Etoposide Impurity 9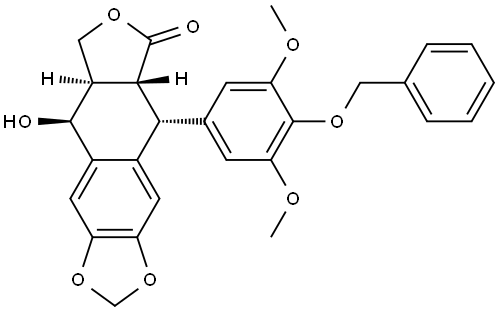
403642-80-8
-
Darunavir Amine dimer impurity
-
methyl[(naphthalen-1-yl)methyl]nitrosoamine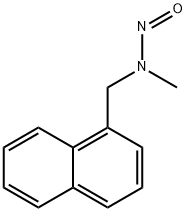
296760-88-8
Cabotegravir Enantiomer Impurity 1309560-64-2
Brivaracetam EP Impurity B/(R,S)-enantiomer of brivaracetam 357336-99-3
Irinotecan Impurity L (HCl Salt) 1992961-26-8
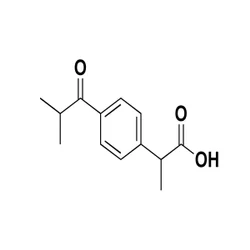
IBUPROFEN IMPURITY L 53949-53-4
Carvedilol EP Impurity B 918903-20-5