Supplier Type
- Manufacturer
- Reagent
- Trader
- contract manufacturer
- custom synthesis
Supplier Region
- Gujarat(24)
- Bangalore(2)
- Pune(5)
- Maharashtra(23)
- West Bengal(1)
- Mumbai(35)
- Andhra Pradesh(2)
- Hyderabad(19)
- Delhi(6)
- Vadodara(6)
- New Delhi(3)
- Nagpur(2)
- Ahmedabad(7)
- Karnataka(7)
- Kerela(1)
- Beijing(2)
- Punjab(3)
- Haryana(3)
- Telangana(2)
- Kolkata(2)
- Madhya Pradesh(1)
- Rajasthan(2)
Purity
- Salicylic Acid
- Rasagiline Mesylate Impurity C
- NLT 99%
- NLT 98%
- NLT 95%
- NLT 90%
- More than 98%
- more than 95%
- more than 90%
- MINIMUM 99%
- Min 98 %
- Mesalazine EP Impurity H
- Mathadone (M225865) impurity. Used in synthesis of methadone, antispasmodics and other pharmaceutica
- Magnesium content 8.49% to 8.84%
- Greater than 99%
- Empagliflozin intermediate, Dapagliflozin Impurity
- Dipropargyl r- amino indane
- Dapagliflozin Intermediate, Dapagliflozin Impurity
- CLASS A
- API Impurity / In-house working standard
- Acetylsalicylic Acid EP Impurity C
- Above 80%
- 99.99%
- 99.99
- 99.98%
- 99.95%
- 99.91%
- 99.90%
- 99.9%
- 99.9
- 99.86%
- 99.85%
- 99.80%
- 99.8%
- 99.73%
- 99.50%
- 99.5%
- 99.42%
- 99.40%
- 99.29%
- 99.10%
- 99.00%
- 99.0%
- 99% Pure
- 99% minimum
- 99% min
- > 99%
- >99%
- 99%
- 99 %+
- 99 %
- 99
- 98=%
- 98+
- 98.60%
- 98.55%
- 98.5 to 101.5%
- 98.5
- 98.12%
- 98.00%
- 98.0% to 101.0%
- 98.0 % -102.0 %
- 98% Min.
- 98% Min
- >98% / >99%
- 98%
- >98%
- 98 % min
- 97-99%
- 97.57%
- 97.53%
- > 97.5 %
- 97.00%
- 97% min
- 97%
- >97%
- 97 %
- 96.80%
- > 96.5 %
- 96.5 - 103.5 %
- 96.39%
- 96.14%
- 96%
- 95-99%
- 95-98%
- 95-97%
- 95.48%
- 95.00%
- 95% min
- 95% DYE CONTENT
- >95%
- 95%
- 94.25%
- 94.00%
- 93 %- 101%
- >92%
- 90%
- >90%
- 90 % Above
- 89%
- 80% Min
- 100%
- 1.0M
- 0.5M/1.0M
- 0.04%
- (R)-N,N-di(prop-2-yn-1-yl)-2,3- dihydro-1
Package
- 25mg
- 100mg
- 250mg
- 500mg
- 1gm
- 50
- 50mg
- 25
- 200
- 100
- 2.5501e+00925 MG 50 MG 100 MG 250 MG EXTRA
- 1g
- 5g
- 10g
- 25g
- 50g
- 100ml
- 100g
- 500ml
- 500g
- 1L
- 1kg
- 5kg
- 10kg
- 25kg
- 50kg
- 100kg
- 500kg
- 1and gm
- 1MT
- 25Kgs
- 250kgs
- 250250 mg.
-
9-(2-Hydroxyethyl)guanine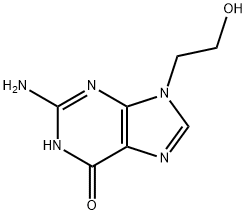
23169-33-7
-
4-Chloroaniline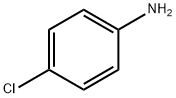
106-47-8
-
Atorvastatin Acetonide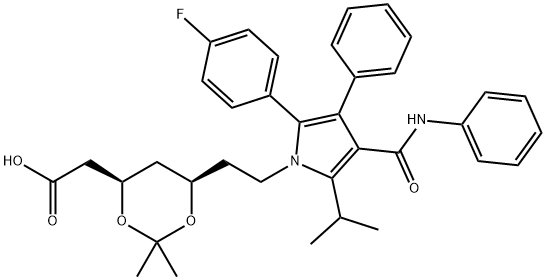
581772-29-4
-
QUETIAPINE EP IMPURITY P / N-ETHYL QUETIAPINE HCL
-
98% Atenolol EP Impurity A or p-hydroxyphenylacetamide, 25mg
-
GDZISNMJFAJFMR-BEQCQUFHSA-M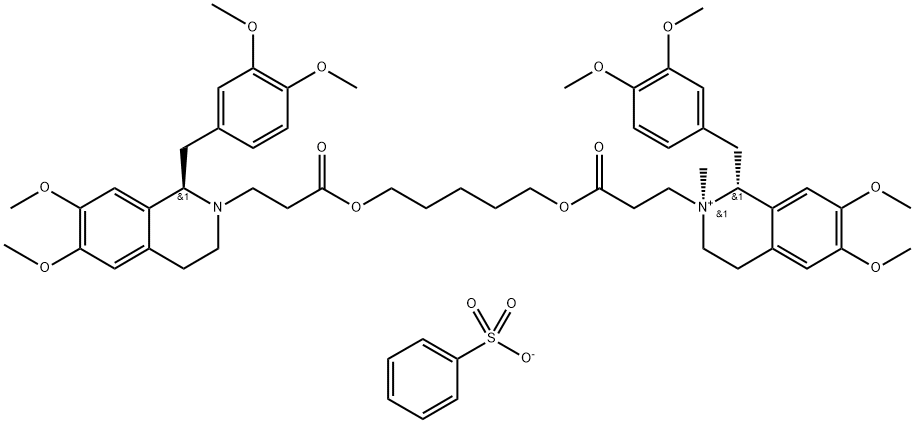
2024603-92-5
-
Oxalic acid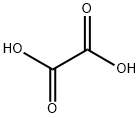
144-62-7
-
Sodium acetate
127-09-3
-
Ammonium nitrate
6484-52-2
-
Carbon tetrachloride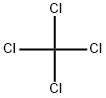
56-23-5
ACICYLOVIR EP IMPURITY P 23169-33-7
Atorvastatin ep impurity P 581772-29-4
98% Atenolol EP Impurity A or p-hydroxyphenylacetamide, 25mg 17194-82-0
Cisatracurium Besylate EP Impurity P 2024603-92-5

Oxalic acid 144-62-7

p-Methoxybenzaldehyde dimethylacetal 2186-92-7

Tris-(p-tolyl)-phosphine 1038-95-5

Tetrachloro-p-benzoquinone 118-75-2

p-Cresol 106-44-5

N-(1,3-Dimethylbutyl)-N'-phenyl-p-phenylenediamine 793-24-8





