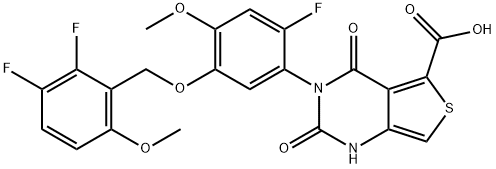
CHEMICAL AND PHYSICAL PROPERTIES
| Solubility | Slightly soluble |
|---|
COMPUTED DESCRIPTORS
| Molecular Weight | 508.4 g/mol |
|---|---|
| XLogP3 | 3.4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 7 |
| Exact Mass | 508.05520648 g/mol |
| Monoisotopic Mass | 508.05520648 g/mol |
| Topological Polar Surface Area | 143 Ų |
| Heavy Atom Count | 35 |
| Formal Charge | 0 |
| Complexity | 826 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Linzagolix is a non-peptide, selective antagonist of the gonadotropin-releasing hormone (GnRH) receptor. It has been studied for the treatment of estrogen-dependent conditions such as uterine fibroids and endometriosis. It is similar to other GnRH receptor antagonists like [cetrorelix], [relugolix], and [elagolix]. Uterine fibroids occur in >70% of women of reproductive age, and when symptomatic are associated with heavy menstrual bleeding, anemia, abdominal pain and pressure, bloating, increased urinary frequency, and reproductive dysfunction. As these fibroids are essentially estrogen-dependent phenomena, hormone therapies which suppress estrogen activity - including GnRH receptor antagonists like linzagolix - are thought to be beneficial by preventing intramyometrial growths in the endometrial glands. Linzagolix was approved for use in the European Union in June 2022 for the management of symptoms caused by uterine fibroids.