814-68-6
Product Name:
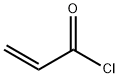
Acryloyl chloride
Formula:
C3H3ClO
Synonyms:
2-Propenoyl chloride;Acrylic acid chloride;Acryloyl chloride
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Acrylyl chloride appears as a liquid. Boiling point 75 °C. Used to make plastics. Polymerization in a closed container can cause pressurization and explosive rupture. (EPA, 1998) |
|---|---|
| Color/Form | Liquid |
| Boiling Point | 167 °F at 760 mmHg (EPA, 1998) |
| Flash Point | 14 °C (57 °F) - closed cup |
| Solubility | Soluble in chlorinated solvents |
| Density | 1.1136 at 68 °F (EPA, 1998) - Denser than water; will sink |
| Vapor Density | 3.63 (Air = 1) |
| Vapor Pressure | 93.0 [mmHg] |
| Stability/Shelf Life | Stable under recommended storage conditions. Contains the following stabilizer(s): Phenothiazine (400 ppm) |
| Decomposition | Hazardous decomposition products formed under fire conditions: Carbon oxides, hydrogen chloride gas. |
| Corrosivity | Corrosive to metals |
| Polymerization | A 500 mL bottle of the acid chloride stabilized with 0.5% of phenothiazine was shipped to a hot climate without refrigeration, and was stored on arrival in a fume cupboard for 2 days at temperatures approaching 50 °C. The material polymerized, bursting the bottle and forming a solid foam. |
| Refractive Index | Index of refraction: 1.4343 at 20 °C/D |
| Other Experimental Properties | Hydrolyzed by water |
| Chemical Classes | Toxic Gases & Vapors -> Acid Halides |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H225:Flammable liquids H290:Corrosive to Metals H302:Acute toxicity,oral H314:Skin corrosion/irritation H330:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 90.51 g/mol |
|---|---|
| XLogP3 | 1.4 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 1 |
| Exact Mass | 89.9872424 g/mol |
| Monoisotopic Mass | 89.9872424 g/mol |
| Topological Polar Surface Area | 17.1 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 57.9 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Acrylyl chloride appears as a liquid. Boiling point 75 °C. Used to make plastics. Polymerization in a closed container can cause pressurization and explosive rupture. (EPA, 1998)
