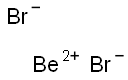CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Very hygroscopic solid; [Merck Index] White odorless powder; [MSDSonline] White needles; [Ullmann] |
|---|---|
| Chemical Classes | Metals -> Beryllium Compounds, Inorganic |
COMPUTED DESCRIPTORS
| Molecular Weight | 168.82 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 168.84681 g/mol |
| Monoisotopic Mass | 166.84886 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 3 |
| Formal Charge | 0 |
| Complexity | 0 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 3 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Beryllium bromide is a bromide of beryllium. Beryllium is a lightweight alkaline earth metal with the atomic number 4. It is a relatively rare element found naturally only combined with other elements in minerals. Bromine is a halogen element with the symbol Br and atomic number 35. Diatomic bromine does not occur naturally, but bromine salts can be found in crustal rock. (L625, L24)