7757-82-6
Product Name:
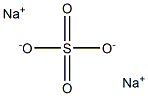
Sodium sulfate
Formula:
Na2SO4
Synonyms:
Disodium Sulfate;Natrii sulfas anhydricus;Sodium sulfate;Sodium Sulfate Anhydrous
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Dry Powder; Dry Powder, Liquid, Other Solid; Dry Powder, Other Solid; Dry Powder, Pellets or Large Crystals; Dry Powder, Pellets or Large Crystals, Liquid; Dry Powder, Water or Solvent Wet Solid; Gas or Vapor; Liquid; Liquid, Other Solid; Other Solid; Pellets or Large Crystals; Water or Solvent Wet Solid |
|---|---|
| Color/Form | Powder or orthorhombic bipyramidal crystals |
| Odor | Odorless |
| Taste | Bitter saline taste |
| Melting Point | 884 °C |
| Solubility | In water, 28.1 g/100 g water at 25 °C |
| Density | 2.671 |
| Stability/Shelf Life | Stable under recommended storage conditions. |
| Decomposition | When heated to decomposition it emits toxic fumes of sulfur oxides and sodium oxide. |
| Viscosity | 2.48 (22% solution at 20 °C) |
| Corrosivity | The rates of corrosion of iron and steel in water are a function of the specific mineral quality as well as the alkalinity and pH values. Sodium sulfate ... is a strong contributor to the rate of corrosion. For example, in water with 400 mg/l of alkalinity (as CaCO3) at pH 7, the corrosion rate will be zero at 200 mg/l of Na2SO4, but when the concentration of sodium sulfate is 400 mg/l, the corrosion rate will be about 100 mg per square cm per day. |
| pH | Neutral or slightly alkaline to litmus paper (5 % solution) |
| Refractive Index | 4.76 g/100 cc water at 0 °C, 42.7 g/100 cc water at 100 °C; soluble in glycerol; insoluble in alcohol; index of refraction: 1.484, 1.477, 1.471 /Natural thenardite/ |
| Dissociation Constants | Sodium sulphate dissociates into Na+ and SO4- ions in water. |
| Relative Evaporation Rate | Evaporation at 20 °C is negligible |
| Other Experimental Properties | Transition point to anhydrous form is 24.4 °C; white, rhombic or tetragonal; 19.5 g/100 cc water at 0 °C, 44 g/100 cc water at 20 °C/Sodium sulfate heptahydrate/ |
| Chemical Classes | Other Classes -> Sulfur Compounds |
COMPUTED DESCRIPTORS
| Molecular Weight | 142.04 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 141.93126821 g/mol |
| Monoisotopic Mass | 141.93126821 g/mol |
| Topological Polar Surface Area | 88.6 Ų |
| Heavy Atom Count | 7 |
| Formal Charge | 0 |
| Complexity | 62.2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 3 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Sodium sulfate (Na₂SO₄) is a highly water-soluble, neutral, and low-cost inorganic salt, commonly found in its anhydrous form and decahydrate (sodium sulfate).
RELATED SUPPLIERS
All suppliers(51)
