CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | CrO3: Dark-red, odorless flakes or powder. [Note: Often used in an aqueous solution (H2CrO4); [NIOSH] Dark, red-purple solid; Soluble in water; [ACGIH] |
|---|---|
| Color/Form | Dark purplish-red crystals /Anhydrous chromic acid/ |
| Boiling Point | Decomposes at about 250 °C /Anhydrous chromic acid, chromic trioxide/ |
| Melting Point | 196 °C /Anhydrous chromic acid/ |
| Solubility | In water, 1.1X10+6 mg/L at 17 °C |
| Density | 1.67-2.82 /Anhydrous chromic acid/ |
| Decomposition | When heated to decomposition it emits smoke and irritating fumes. |
| Corrosivity | Attacks most metals, particularly copper and brass. Attacks cloth, leather, and some plastics. |
| Dissociation Constants | pKa = -0.8 to 1.6 |
| Other Experimental Properties | Weighs 13.9 lb per gal /Chromic acid soln/ |
| Chemical Classes | Metals -> Chromium Compounds, Inorganic |
COMPUTED DESCRIPTORS
| Molecular Weight | 118.010 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 117.935813 g/mol |
| Monoisotopic Mass | 117.935813 g/mol |
| Topological Polar Surface Area | 74.6 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 81.3 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
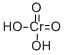
Chromic acid is a chromium oxoacid. It has a role as an oxidising agent. It is a conjugate acid of a hydrogenchromate.