7637-07-2
Product Name:
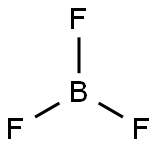
Boron trifluoride
Formula:
BF3
Synonyms:
Boron fluoride;Boron trifluoride;Trifluoroborane;Trifluoroboron
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Boron trifluoride is a colorless gas with a pungent odor. It is toxic by inhalation. It is soluble in water and slowly hydrolyzed by cold water to give off hydrofluoric acid, a corrosive material. Its vapors are heavier than air. Prolonged exposure of the containers to fire or heat may result in their violent rupturing and rocketing. |
|---|---|
| Color/Form | Colorless gas |
| Odor | Pungent, suffocating odor |
| Boiling Point | -148 °F at 760 mmHg (NIOSH, 2023) |
| Melting Point | -196.1 °F (EPA, 1998) |
| Solubility | 106 % (in cold H2O) (NIOSH, 2023) |
| Density | 1.6 (EPA, 1998) - Denser than water; will sink |
| Vapor Density | 2.4 (EPA, 1998) - Heavier than air; will sink (Relative to Air) |
| Vapor Pressure | 760 mmHg at -149.26 °F Liquid (EPA, 1998) |
| Stability/Shelf Life | Boron trifluoride ... is stable in dry atmospheres. |
| Decomposition | Dangerous; when heated to decomposition or upon contact with water or steam, will produce toxic and corrosive fumes of /hydrogen fluoride/. |
| Viscosity | 0.0171 m Pa.s (gas) at 25 °C |
| Corrosivity | Boron trifluoride will attack some forms of plastics, rubber, and coatings |
| Heat of Vaporization | 19.33 kJ/mol at -101 °C |
| Surface Tension | 17.2 mN/m at -100 °C |
| Ionization Potential | 15.50 eV |
| Polymerization | The substance will polymerize unsaturated compounds. |
| Odor Threshold | Odor Threshold Low: 1.5 [ppm] Odor threshold from AIHA |
| Other Experimental Properties | Forms dense white fumes in moist air; ... reacts with incandescence when heated with alkali metals or alkaline earth metals except magnesium |
| Chemical Classes | Toxic Gases & Vapors -> Corrosive Gases |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Gas Cylinder Compressed Gases GHS04  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H280:Gases under pressure H314:Skin corrosion/irritation H330:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P410+P403:Protect from sunlight. Store in a well-ventilated place. |
COMPUTED DESCRIPTORS
| Molecular Weight | 67.81 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 68.0045147 g/mol |
| Monoisotopic Mass | 68.0045147 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 4 |
| Formal Charge | 0 |
| Complexity | 8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Boron trifluoride is a colorless gas with a pungent odor. It is toxic by inhalation. It is soluble in water and slowly hydrolyzed by cold water to give off hydrofluoric acid, a corrosive material. Its vapors are heavier than air. Prolonged exposure of the containers to fire or heat may result in their violent rupturing and rocketing.
