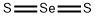CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Selenium disulfide is a bright orange colored powder. It is insoluble in water. It is toxic by ingestion and inhalation, and is irritating to skin and eyes. It is used to make medicated shampoos. |
|---|---|
| Color/Form | Red-yellow crystals |
| Boiling Point | Decomposes (NTP, 1992) |
| Melting Point | less than 212 °F (NTP, 1992) |
| Solubility | 10 to 50 mg/mL at 68 °F (NTP, 1992) |
| Decomposition | When heated to decomposition, it emits very toxic fumes of /sulfur oxides and selenium/. |
| Other Experimental Properties | Decomposes in aqua regia, and in nitric acid |
| Chemical Classes | Other Classes -> Other Inorganic Compounds |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H373:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P314:Get medical advice/attention if you feel unwell. |
COMPUTED DESCRIPTORS
| Molecular Weight | 143.1 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 143.86066 g/mol |
| Monoisotopic Mass | 143.86066 g/mol |
| Topological Polar Surface Area | 64.2 Ų |
| Heavy Atom Count | 3 |
| Formal Charge | 0 |
| Complexity | 18.3 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Selenium disulfide is a bright orange colored powder. It is insoluble in water. It is toxic by ingestion and inhalation, and is irritating to skin and eyes. It is used to make medicated shampoos.