65-28-1
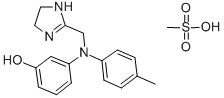
Product Name:
Phentolamine mesilate
Formula:
C18H23N3O4S
Synonyms:
2-(N-[m-Hydroxyphenyl]-p-toluidinomethyl)imidazoline;Phentolamine methanesulfonate salt
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Solubility | >56.6 [ug/mL] (The mean of the results at pH 7.4) |
|---|
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 377.5 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 4 |
| Exact Mass | 377.14092740 g/mol |
| Monoisotopic Mass | 377.14092740 g/mol |
| Topological Polar Surface Area | 111 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 456 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Phentolamine Mesylate is the mesylate salt of a synthetic imidazoline with alpha-adrenergic antagonist activity. As a competitive alpha-adrenergic antagonist, phentolamine binds to alpha-1 and alpha-2 receptors, resulting in a decrease in peripheral vascular resistance and vasodilatation. This agent also may block 5-hydroxytryptamine (5-HT) receptors and stimulate release of histamine from mast cells.
