6106-04-3
Product Name:
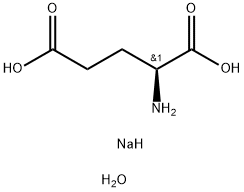
L(+)-Monosodium glutamate monohydrate
Formula:
C5H12NNaO5
Synonyms:
Glu;Monosodium glutamate;MSG;L-Glutamic acid monosodium salt;(S)-2-Aminopentanedioic acid
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Monosodium glutamate appears as white or off-white crystalline powder with a slight peptone-like odor. pH (0.2% solution)7.0. (NTP, 1992) |
|---|---|
| Color/Form | White free flowing crystals or crystalline powder |
| Odor | Practically odorless |
| Taste | Meaty taste comes from contaminants in crude glutamates; sweet-saline taste in large concn; no flavor in small quantity |
| Boiling Point | 225 °C (decomposes) |
| Melting Point | 450 °F (Decomposes) (NTP, 1992) |
| Solubility | greater than or equal to 100 mg/mL at 68 °F (NTP, 1992) |
| Density | 26.2 (saturated water solution at 20 °C) |
| Optical Rotation | [α]D/20 between + 24,8° and + 25,3°; (10 % solution (anhydrous basis) in 2N HCl, 200 mm tube) |
| Decomposition | When heated to decomposition it emits toxic fumes of oxides of /nitrogen and sodium oxide/. |
| pH | Between 6,7 and 7,2 (5 % solution) |
| Other Experimental Properties | THE MONOHYDRATE FORMS NEEDLES |
| Chemical Classes | Nitrogen Compounds -> Other Nitrogen Compounds |
COMPUTED DESCRIPTORS
| Molecular Weight | 187.13 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 4 |
| Exact Mass | 187.04566670 g/mol |
| Monoisotopic Mass | 187.04566670 g/mol |
| Topological Polar Surface Area | 104 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 149 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 3 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Monosodium glutamate appears as white or off-white crystalline powder with a slight peptone-like odor. pH (0.2% solution)7.0. (NTP, 1992)
