CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid |
|---|---|
| Color/Form | White prisms from alcohol-diethyl ether; needles from petroleum ether |
| Boiling Point | Sublimes at 135 °C |
| Melting Point | 226 (dec) °C |
| Solubility | In water, 3.733X10-5 mg/L at 37.5 °C |
| Density | 1.337 g/cu cm at 20 °C |
| LogP | 3 |
| Stability/Shelf Life | SENSITIVE TO LIGHT & MOISTURE /papaverine/ |
| Decomposition | When heated to decomposition it emits toxic fumes of /oxides of nitrogen/. |
| pH | Optimal pH for storage of papverine solutions: 2.0-2.8 |
| Refractive Index | Index of refraction: 1.625 C/D |
| Dissociation Constants | pKa = 8.07 at 25 °C |
| Collision Cross Section | 185.3 Ų [M+H]+ [CCS Type: TW, Method: Major Mix IMS/Tof Calibration Kit (Waters)] |
| Kovats Retention Index | 2825 2824 2814 2800 2819 2818 2815 2808 2840 2806 2825 2825 2820 2813 2825 2780 2815 2825 2770 |
| Other Experimental Properties | Monoclinic rods from water, mp 220-225 °C. UV max (ethanol) 249-250, 280-282, 311 nm (log E 4.69, 3.80, 3.82). One gram dissolves in about 40 mL water. Soluble in alcohol and chloroform. Practically insoluble in ether. pH of 0.05 molar solution 3.9; pH pf 2% aqueous solution 3.3 /Papverine hydrochloride/ |
COMPUTED DESCRIPTORS
| Molecular Weight | 339.4 g/mol |
|---|---|
| XLogP3 | 3.9 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 6 |
| Exact Mass | 339.14705815 g/mol |
| Monoisotopic Mass | 339.14705815 g/mol |
| Topological Polar Surface Area | 49.8 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 407 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
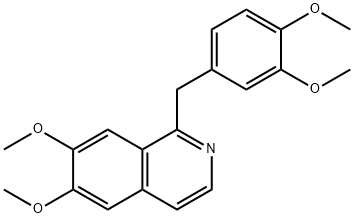
Papaverine is a benzylisoquinoline alkaloid that is isoquinoline substituted by methoxy groups at positions 6 and 7 and a 3,4-dimethoxybenzyl group at position 1. It has been isolated from Papaver somniferum. It has a role as a vasodilator agent and an antispasmodic drug. It is a benzylisoquinoline alkaloid, a member of isoquinolines and a dimethoxybenzene.