53963-43-2
Product Name:
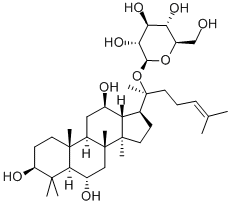
GINSENOSIDEF1
Formula:
C36H62O9
Synonyms:
(3β,6α,12β)-3,6,12-trihydroxydammar-24-en-20-yl β-D-glucopyranoside;20(S)-ginsenoside F1;Ginsenoside F1;panaxoside A progenin
Inquiry
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P405:Store locked up. P403+P233:Store in a well-ventilated place. Keep container tightly closed. P501:Dispose of contents/container to..… |
COMPUTED DESCRIPTORS
| Molecular Weight | 638.9 g/mol |
|---|---|
| XLogP3 | 4.3 |
| Hydrogen Bond Donor Count | 7 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 7 |
| Exact Mass | 638.43938355 g/mol |
| Monoisotopic Mass | 638.43938355 g/mol |
| Topological Polar Surface Area | 160 Ų |
| Heavy Atom Count | 45 |
| Formal Charge | 0 |
| Complexity | 1110 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 16 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Ginsenoside F1 is a ginsenoside found in Panax species that is dammarane which is substituted by hydroxy groups at the 3beta, 6alpha, 12beta and 20 pro-S positions, in which the hydroxy group at position 20 has been converted to the corresponding beta-D-glucopyranoside, and in which a double bond has been introduced at the 24-25 position. It has a role as a plant metabolite and an apoptosis inhibitor. It is a 12beta-hydroxy steroid, a 3beta-hydroxy steroid, a beta-D-glucoside, a ginsenoside, a tetracyclic triterpenoid, a 6alpha-hydroxy steroid and a 3beta-hydroxy-4,4-dimethylsteroid. It derives from a hydride of a dammarane.
