487-89-8
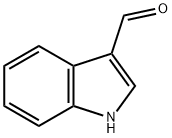
Product Name:
Indole-3-carboxaldehyde
Formula:
C9H7NO
Synonyms:
3-Formylindole;Indole-3-carbaldehyde;3-Indolylformaldehyde;Indol-3-aldehyde;β-Indolylaldehyde
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Tan powder; [Alfa Aesar MSDS] |
|---|---|
| Melting Point | 197 - 199 °C |
| Vapor Pressure | 0.00043 [mmHg] |
| LogP | 1.68 |
| Kovats Retention Index | 1786.9 |
| Chemical Classes | Nitrogen Compounds -> Indoles |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P363:Wash contaminated clothing before reuse. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P405:Store locked up. |
COMPUTED DESCRIPTORS
| Molecular Weight | 145.16 g/mol |
|---|---|
| XLogP3 | 1.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 1 |
| Exact Mass | 145.052763847 g/mol |
| Monoisotopic Mass | 145.052763847 g/mol |
| Topological Polar Surface Area | 32.9 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 158 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Indole-3-carbaldehyde is a heteroarenecarbaldehyde that is indole in which the hydrogen at position 3 has been replaced by a formyl group. It has a role as a plant metabolite, a human xenobiotic metabolite, a bacterial metabolite and a marine metabolite. It is a heteroarenecarbaldehyde, an indole alkaloid and a member of indoles.
RELATED SUPPLIERS
All suppliers(10)
