CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Dry Powder; Other Solid |
|---|---|
| Color/Form | Crystals |
| Odor | Odorless |
| Melting Point | 228.5 °C |
| Solubility | 11000 mg/L (at 25 °C) |
| Vapor Pressure | 0.0000061 [mmHg] |
| Dissociation Constants | 2.43 |
| Collision Cross Section | 130.5 Ų [M+H-H2O]+ [CCS Type: DT, Method: single field calibrated with Agilent tune mix (Agilent)] |
| Other Experimental Properties | 190 °C when rapidly heated, with decomposition into CO2 and nicotinic acid |
| Chemical Classes | Nitrogen Compounds -> Amino Carboxylic Acids |
COMPUTED DESCRIPTORS
| Molecular Weight | 167.12 g/mol |
|---|---|
| XLogP3 | 0.2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 2 |
| Exact Mass | 167.02185764 g/mol |
| Monoisotopic Mass | 167.02185764 g/mol |
| Topological Polar Surface Area | 87.5 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 204 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
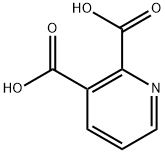
Quinolinic acid is a pyridinedicarboxylic acid that is pyridine substituted by carboxy groups at positions 2 and 3. It is a metabolite of tryptophan. It has a role as a NMDA receptor agonist, a human metabolite, a mouse metabolite and an Escherichia coli metabolite. It is a conjugate acid of a quinolinate(1-) and a quinolinate.