33419-42-0
Product Name:
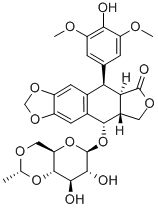
Etoposide
Formula:
C29H32O13
Synonyms:
4′-Demethylepipodophyllotoxin 9-(4,6-O-ethylidene-β-D -glucopyranoside);Etoposide;VP16;VP-16;
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid |
|---|---|
| Color/Form | Crystals from methanol |
| Melting Point | 236-251 °C |
| Solubility | Sparingly soluble |
| Vapor Pressure | 5.4X10-23 mm Hg at 25 °C /Estimated/ |
| LogP | 0.6 |
| Henry's Law Constant | Henry's Law constant = 1.7X10-30 atm-cu m/mol at 25 °C /Estimated/ |
| Optical Rotation | Specific optical rotation = -110.5 °C @ 20 °C/D ( c= 0.6 in chloroform). |
| Dissociation Constants | pKa = 9.8 |
| Other Experimental Properties | MW: 668.54. Soluble in water. Practically insoluble in organic solvents. /Phosphate/ |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H350:Carcinogenicity H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. |
COMPUTED DESCRIPTORS
| Molecular Weight | 588.6 g/mol |
|---|---|
| XLogP3 | 0.6 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 13 |
| Rotatable Bond Count | 5 |
| Exact Mass | 588.18429107 g/mol |
| Monoisotopic Mass | 588.18429107 g/mol |
| Topological Polar Surface Area | 161 Ų |
| Heavy Atom Count | 42 |
| Formal Charge | 0 |
| Complexity | 969 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 10 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Etoposide is a beta-D-glucoside, a furonaphthodioxole and an organic heterotetracyclic compound. It has a role as an antineoplastic agent and a DNA synthesis inhibitor. It is functionally related to a podophyllotoxin and a 4'-demethylepipodophyllotoxin.
