2949-92-0
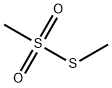
Product Name:
S-Methyl methanethiolsulfonate
Formula:
C2H6O2S2
Synonyms:
S-Methyl thiomethanesulfonate;MMTS
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Clear yellow liquid with a stench; [Sigma-Aldrich MSDS] |
|---|---|
| Boiling Point | 266.00 to 267.00 °C. @ 760.00 mm Hg |
| Solubility | slightly |
| Kovats Retention Index | 1092 |
| Chemical Classes | Other Classes -> Sulfur Compounds |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H227:Flammable liquids H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P405:Store locked up. P403+P233:Store in a well-ventilated place. Keep container tightly closed. P501:Dispose of contents/container to..… |
COMPUTED DESCRIPTORS
| Molecular Weight | 126.20 g/mol |
|---|---|
| XLogP3 | 0 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | 125.98092178 g/mol |
| Monoisotopic Mass | 125.98092178 g/mol |
| Topological Polar Surface Area | 67.8 Ų |
| Heavy Atom Count | 6 |
| Formal Charge | 0 |
| Complexity | 106 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
S-methyl methanethiosulfonate is a sulfonic acid derivative obtained by condensaton of methanesulfonic acid with methanethiol. It has a role as a metabolite. It is a sulfonic acid derivative and a thiosulfonate ester. It is functionally related to a methanesulfonic acid and a methanethiol.
