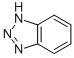
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | 1,2,3-benzotriazole appears as white to light tan crystals or white powder. No odor. (NTP, 1992) |
|---|---|
| Color/Form | Needles from chloroform or benzene |
| Odor | No odor |
| Boiling Point | 399 °F at 15 mmHg (NTP, 1992) |
| Melting Point | 208 to 212 °F (NTP, 1992) |
| Flash Point | 170 °C (338 °F) - closed cup |
| Solubility | 1 to 5 mg/mL at 74.7 °F (NTP, 1992) |
| Density | 1.36 g/cu cm at 20 °C |
| Vapor Density | 4.1 (Air = 1) |
| Vapor Pressure | Vapor pressure: <0.01 kPa at 25 °C |
| LogP | log Kow= 1.44 |
| Autoignition Temperature | 210 °C |
| Decomposition | Hazardous decomposition products formed under fire conditions - Carbon oxides, nitrogen oxides (NOx). |
| pH | The solution in water is a weak acid |
| Dissociation Constants | pKa = 8.37 at 20 °C |
| Collision Cross Section | 118.3 Ų [M-H]- 122.42 Ų [M+H]+ |
| Kovats Retention Index | 1469 |
| Other Experimental Properties | Can exist in 2 tautomeric forms |
| Chemical Classes | Nitrogen Compounds -> Triazoles |
COMPUTED DESCRIPTORS
| Molecular Weight | 119.12 g/mol |
|---|---|
| XLogP3 | 1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 119.048347172 g/mol |
| Monoisotopic Mass | 119.048347172 g/mol |
| Topological Polar Surface Area | 41.6 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 92.5 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
1,2,3-benzotriazole appears as white to light tan crystals or white powder. No odor. (NTP, 1992)