CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | DL- and L-forms are solids; [Merck Index] White crystalline solid; [Sigma-Aldrich MSDS] |
|---|---|
| Color/Form | Transparent, hexagonal sheets or plates; metallic luster of crystals |
| Melting Point | 265 °C (decomposes) |
| Vapor Pressure | 0.00000008 [mmHg] |
| Decomposition | When heated to decomposition it emits very toxic fumes of /nitrogen oxides and selenium/. |
| Collision Cross Section | 135.2 Ų [M+H]+ [CCS Type: DT, Method: single field calibrated with ESI Low Concentration Tuning Mix (Agilent)] |
| Other Experimental Properties | Selenium analog of methionine |
| Chemical Classes | Biological Agents -> Amino Acids and Derivatives |
COMPUTED DESCRIPTORS
| Molecular Weight | 196.12 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 4 |
| Exact Mass | 196.99550 g/mol |
| Monoisotopic Mass | 196.99550 g/mol |
| Topological Polar Surface Area | 63.3 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 97 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
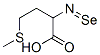
Selenomethionine is a selenoamino acid that is the selenium analogue of methionine. It has a role as a plant metabolite. It is a member of selenomethionines and a selenoamino acid.