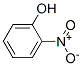
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | 2-nitrophenol is a yellow solid. Sinks in and mixes slowly with water. (USCG, 1999) |
|---|---|
| Color/Form | Light yellow needles or prisms |
| Odor | Peculiar, aromatic color |
| Taste | SWEET TASTE |
| Boiling Point | 417 to 421 °F at 760 mmHg (Decomposes) (NTP, 1992) |
| Melting Point | 113 to 115 °F (NTP, 1992) |
| Flash Point | 108 °C c.c. |
| Solubility | less than 1 mg/mL at 70 °F (NTP, 1992) |
| Density | 1.49 at 68 °F (USCG, 1999) - Denser than water; will sink |
| Vapor Pressure | 1 mmHg at 120.7 °F (NTP, 1992) |
| LogP | log Kow = 1.79 |
| Henry's Law Constant | Henry's Law constant = 1.3X10-5 atm-cu m/mol at 20 °C |
| Autoignition Temperature | 550 °C |
| Decomposition | When heated to decomp it emits toxic fumes of /nitrogen oxides/. |
| Heat of Combustion | -8,912 Btu/lb = -4,950 cal/g = -207X10+5 J/kg |
| Heat of Vaporization | 12,497.3 g cal/g mole |
| Ionization Efficiency | Negative |
| Odor Threshold | Detection in air: 8.0X10-11 moles/cu m |
| Refractive Index | Index of refraction: 1.5723 at 50 °C/D |
| Dissociation Constants | pKa = 7.230 at 25 °C |
| Kovats Retention Index | 1099 1149 1166 1149 1147.1 |
| Other Experimental Properties | Heat of fusion: 17.44 kJ/mol |
| Chemical Classes | Nitrogen Compounds -> Nitrophenols |
COMPUTED DESCRIPTORS
| Molecular Weight | 139.11 g/mol |
|---|---|
| XLogP3 | 1.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 139.026943022 g/mol |
| Monoisotopic Mass | 139.026943022 g/mol |
| Topological Polar Surface Area | 66 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 131 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
2-nitrophenol is a yellow solid. Sinks in and mixes slowly with water. (USCG, 1999)