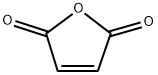
| Physical Description |
Maleic anhydride appears as colorless crystalline needles, flakes, pellets, rods, briquettes, lumps or a fused mass. Melts at 113 °F. Shipped both as a solid and in the molten state. Vapors, fumes and dusts strong irritate the eyes, skin and mucous membranes. Flash point 218 °F. Autoignition temperature 890 °F. Used to make paints and plastics and other chemicals. |
| Color/Form |
Orthorhombic needles from chloroform; commercial grades furnished in fused form, as briquettes |
| Odor |
Pungent odor |
| Boiling Point |
387 to 390 °F at 760 mmHg (NTP, 1992) |
| Melting Point |
127 °F (NTP, 1992) |
| Flash Point |
218 °F (NTP, 1992) |
| Solubility |
Soluble; decomposes in hot solvent (NTP, 1992) |
| Density |
1.43 at 59 °F (USCG, 1999) - Denser than water; will sink |
| Vapor Density |
3.38 (Air = 1) |
| Vapor Pressure |
0.2 mmHg (NIOSH, 2023) |
| LogP |
log Kow = 1.62 |
| Stability/Shelf Life |
Stable under normal laboratory storage conditions. |
| Autoignition Temperature |
878 °F (USCG, 1999) |
| Decomposition |
Maleic anhydride decomposes exothermically, evolving carbon dioxide in the presence of dimethylamine, triethylamine, pyridine, or quinoline at temperatures above 150 °C. |
| Viscosity |
Viscosity in mPa.s (cP): 0.61 at 60 °C; 1.07 at 90 °C; 0.6 at 150 °C |
| Corrosivity |
Corrosive material. |
| Heat of Combustion |
-1389.5 kJ/mol |
| Heat of Vaporization |
54.8 kJ/mol |
| pH |
pH of water solutions: 2.42 at 1X10-2 M; 2.62 at 5X10-3 M; 3.10 at 1X10-4 M |
| Ionization Potential |
9.90 eV |
| Odor Threshold |
Odor Threshold Low: 0.25 [mmHg] Odor Threshold High: 0.32 [mmHg] Odor threshold from AIHA |
| Kovats Retention Index |
791 807 790 |
| Other Experimental Properties |
Specific heat: 0.285 (solid); 0.396 (liquid). |
| Chemical Classes |
Plastics & Rubber -> Acid Anhydrides, Cyclic |