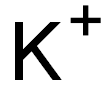CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid |
|---|---|
| Boiling Point | 1689 °C (potassium sulfate) |
| Melting Point | 1067 °C (potassium sulfate) |
| Solubility | 110 g/l (20°C) (potassium sulfate) |
COMPUTED DESCRIPTORS
| Molecular Weight | 39.0983 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 0 |
| Rotatable Bond Count | 0 |
| Exact Mass | 38.96370648 g/mol |
| Monoisotopic Mass | 38.96370648 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 1 |
| Formal Charge | 1 |
| Complexity | 0 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Potassium(1+) is a monoatomic monocation obtained from potassium. It has a role as a human metabolite and a cofactor. It is an alkali metal cation, an elemental potassium, a monovalent inorganic cation and a monoatomic monocation.