CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid |
|---|---|
| Color/Form | LEAFLETS FROM ALCOHOL |
| Odor | FAINT, CHARACTERISTIC |
| Boiling Point | 280 °C WITH SOME DECOMP |
| Melting Point | 143-146 °C |
| Solubility | Freely soluble |
| LogP | log Kow = -0.34 |
| Stability/Shelf Life | SENSITIVE TO LIGHT |
| Decomposition | When heated to decomposition it emits very toxic fumes of nitroxides and sulfoxides. |
| pH | SOLN ARE PRACTICALLY NEUTRAL TO LITMUS |
| Collision Cross Section | 117.2 Ų [M+H]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards] |
COMPUTED DESCRIPTORS
| Molecular Weight | 114.17 g/mol |
|---|---|
| XLogP3 | -0.3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 114.02516937 g/mol |
| Monoisotopic Mass | 114.02516937 g/mol |
| Topological Polar Surface Area | 47.4 Ų |
| Heavy Atom Count | 7 |
| Formal Charge | 0 |
| Complexity | 119 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
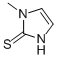
Methimazole is a member of the class of imidazoles that it imidazole-2-thione in which a methyl group replaces the hydrogen which is attached to a nitrogen. It has a role as an antithyroid drug.