22260-51-1
Product Name:
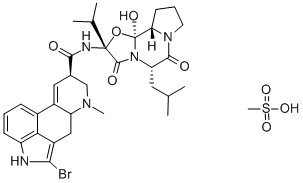
Bromocriptine mesylate
Formula:
C33H44BrN5O8S
Synonyms:
(+)-2-Bromo-12′-hydroxy-2′-(1-methylethyl)-5′-(2-methylpropyl)ergotaman-3′,6′-18-trione methanesulfonate salt;(+)-Bromocriptine methanesulfonate salt;2-Bromo-α-ergocryptine methanesulfonate salt;Bromocriptine mesylate salt
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Collision Cross Section | 244.4 Ų [M+H]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards] |
|---|
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. |
COMPUTED DESCRIPTORS
| Molecular Weight | 750.7 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 5 |
| Exact Mass | 749.20940 g/mol |
| Monoisotopic Mass | 749.20940 g/mol |
| Topological Polar Surface Area | 181 Ų |
| Heavy Atom Count | 48 |
| Formal Charge | 0 |
| Complexity | 1320 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Bromocriptine methanesulfonate is a methanesulfonate salt. It has a role as an antiparkinson drug. It contains a bromocriptine.
