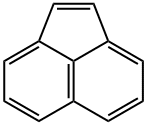
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Acenaphthylene is a colorless crystalline solid. Insoluble in water. Used in dye synthesis, insecticides, fungicides, and in the manufacture of plastics. |
|---|---|
| Color/Form | Yellow needles |
| Boiling Point | 509 to 527 °F at 760 mmHg (NTP, 1992) |
| Melting Point | 200.3 to 202.1 °F (NTP, 1992) |
| Flash Point | 122.0 °C (251.6 °F) - closed cup |
| Solubility | Insoluble (NTP, 1992) |
| Density | 0.8988 at 61 °F (NTP, 1992) - Less dense than water; will float |
| Vapor Pressure | 0.00668 [mmHg] |
| LogP | log Kow = 3.93 |
| Henry's Law Constant | Henry's Law constant = 1.25X10-4 atm-cu m/mol at 25 °C |
| Stability/Shelf Life | Stable under recommended storage conditions. |
| Decomposition | Hazardous decomposition products formed under fire conditions - Carbon oxides. |
| Heat of Vaporization | 64.6-69.1 kJ/mol |
| Collision Cross Section | 125.71 Ų [M+H]+ [CCS Type: DT, Method: stepped-field] |
| Kovats Retention Index | 1418.54 1460 1419 1408.9 1409 1424.62 1457.55 1495 1433 1434 1434 1445 1422 1428 1403 1421.4 244.6 247.4 247.7 244 |
| Other Experimental Properties | Enthalpy of sublimation, 17.0 kcal/mol at 298 K |
| Chemical Classes | Other Classes -> Polycyclic Aromatic Hydrocarbons |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 152.19 g/mol |
|---|---|
| XLogP3 | 3.7 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 0 |
| Rotatable Bond Count | 0 |
| Exact Mass | 152.062600255 g/mol |
| Monoisotopic Mass | 152.062600255 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 184 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Acenaphthylene is a colorless crystalline solid. Insoluble in water. Used in dye synthesis, insecticides, fungicides, and in the manufacture of plastics.