1617-53-4
Product Name:
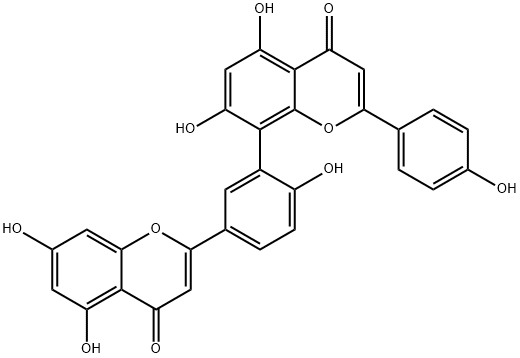
Amentoflavone
Formula:
C30H18O10
Synonyms:
Didemethyl-ginkgetin;I3′,II8-Biapigenin;Tridemethylsciadopitysin
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid |
|---|---|
| Melting Point | 300 °C |
| Collision Cross Section | 230.16 Ų [M+H]+ [CCS Type: DT, Method: stepped-field] |
COMPUTED DESCRIPTORS
| Molecular Weight | 538.5 g/mol |
|---|---|
| XLogP3 | 5 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 3 |
| Exact Mass | 538.08999677 g/mol |
| Monoisotopic Mass | 538.08999677 g/mol |
| Topological Polar Surface Area | 174 Ų |
| Heavy Atom Count | 40 |
| Formal Charge | 0 |
| Complexity | 1050 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Amentoflavone is a biflavonoid that is obtained by oxidative coupling of two molecules of apigenin resulting in a bond between positions C-3 of the hydroxyphenyl ring and C-8 of the chromene ring. A natural product found particularly in Ginkgo biloba and Hypericum perforatum. It has a role as a cathepsin B inhibitor, an antiviral agent, an angiogenesis inhibitor, a P450 inhibitor and a plant metabolite. It is a biflavonoid, a hydroxyflavone and a ring assembly.
