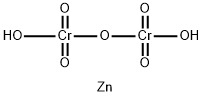
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Zinc dichromate appears as solid orange-yellow crystal or powder. (USCG, 1999) |
|---|---|
| Solubility | Soluble in acids and liquid ammonia |
| Density | 3.4 at 20 °C |
| Autoignition Temperature | Not flammable (USCG, 1999) |
| Decomposition | Decomposes in hot water. |
| pH | ... hexavalent chromium compounds are acidic |
| Other Experimental Properties | Reddish-brown crystal; hygroscopic; mol wt: 335.41; decomposes in hot water; very soluble in cold water; insoluble in alcohol, ether; soluble in acids. /Zinc dichromate trihydrate/ |
| Chemical Classes | Metals -> Chromium Compounds, Inorganic |
COMPUTED DESCRIPTORS
| Molecular Weight | 281.4 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 0 |
| Exact Mass | 279.774554 g/mol |
| Monoisotopic Mass | 279.774554 g/mol |
| Topological Polar Surface Area | 124 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 194 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Zinc dichromate appears as solid orange-yellow crystal or powder. (USCG, 1999)